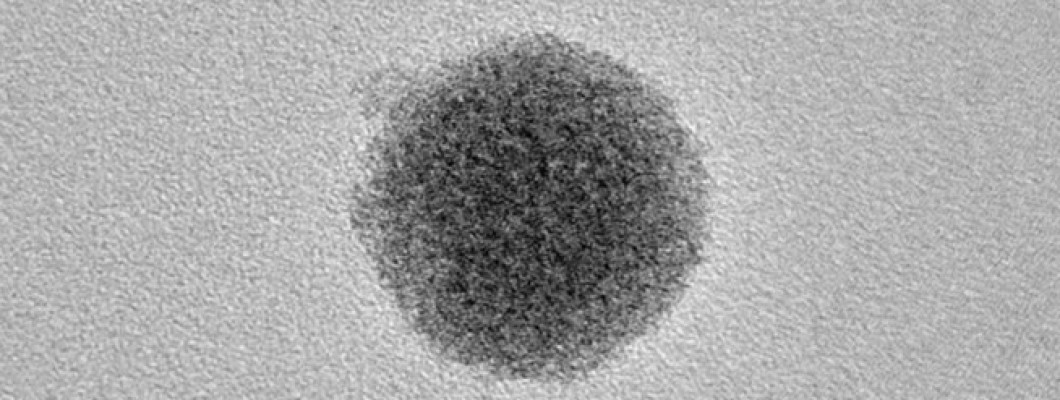
Fullerene
is an allotrope of carbon where its carbon atoms are linked with each other
with the help of single and double bonds. Due to these bones, a partially
closed case-like structure is formed, which is like fused rings containing
several atoms. Fullerene can be called a series of hollow carbon molecules
which formulate a closed cage. Fullerene contains 28 bonds and 12
pentagonal rings, forming the basis of icosahedral symmetry, which is like a
close cage structure. In its natural form, Fullerene is highly symmetrical,
having a similar structure as that of graphite. It is made up of sheets
connected with the hexagonal sheets or the cage structures. However, sometimes
Fullerene contains some pentagonal and has taken heptagonal rings; however,
these structures do not form a sheet to become planar. Because of this
structure, they are often known as Buckyballs and buck tubes which depend on
their structure and shape. However, commonly an infinite number of Fullerene
exists. Fullerene can exist in various forms, for example, C60,
C70, C80, and C90. The existence of these
forms depends on the number of carbon atoms existing in a particular molecule.
Apart from these, the common structure of Fullerene is primarily dependent on
pentagonal and hexagonal rings, which are formed together to form a shape like
icosahedron. The behavior and structure of Fullerene depend on the temperature.
With an increase in temperature, Fullerene is converted into C70.
The structure of Fullerene depends on the available pressure as the pressure
can change its structure in different forms. Fullerene is basically stable but
has some reactive capability as it cannot stay unreactive. It is soluble in
various organic solvents, including toluene, chlorobenzene, and
1,2,3-trichloropropane. Buckyballs and carbon additives act as a building block
for a variety of derivatives and the huge structures of Fullerene. Fullerene is
widely used in various fields, including the medical field, where it has a
light-activated antimicrobial agent. It is also widely used in drug delivery systems.
It acts as a catalyst and is widely used as a lubricant. Because of its
conductivity, it is also used as a conductor. However, some types of Fullerenes
can also be used as the absorbent for different gases. It is also used in the
manufacturing and production of cosmetics. C60-based films are widely used for
photovoltaic applications. Fullerene is also used for the manufacturing and
construction of other carbon nanotubes that are based on fibers and fabrics.





111 Comment(s)
HOKI178 Trusted Gacor Online Slot Site, Favorite of Slot88 Gambling Lovers Your wait for a trusted online slot gambling site is over at Hoki178. You will find all types of gacor slot games today that are easy to leak, besides that you can experience the best service 24 hours a day so that the playing experience feels more complete. We also have official permission as the main distributor for the slot88 site and you will also get live RTP leak information that we have provided on the website display. Several well-known providers who are always known for providing easy-to-win online slot gambling games are fully available for you to use as a place to play. Each trusted Gacor slot site has its own advantages in terms of animation, appearance and other interesting features. So, what are the names of these providers? The following include Pragmatic Play, Slot88, PG Soft, Jili, Microgaming, Habanero, Joker123 and many others. Todays 3 Most Special Gacor Slot Games. Finding todays gacor slot games that can provide continuous jackpot wins is not easy, many players are trapped in confusion when choosing this type of slot machine and ultimately result in defeat. We thought of a solution to prevent you from this problem by presenting a leak of the 3 best online slot gambling games , the most special and most popular among players: 1) Gates of Olympus: Todays gacor slot gambling game is the most phenomenal that has ever existed, its fame comes from the frequent maxwin jackpots awarded amounting to x5 to x500. The games Greek god theme makes it look very unique, playing it is also very easy to understand, making it always the first choice for all players. 2) Sultan Petir Zeus: As the name suggests, Sultan Lightning Zeus has an animation in the form of the god Zeus who is famous for his lightning powers. The only online slot gambling game that can produce thunderous thunder when you win the jackpot and luckily, it usually often gives you winnings in a leaked way which makes the player become a sultan within a day. 3) Starlight Princess: The best online slot game from the provider Pragmatic Play never stops providing surprises, like Starlight Princess is one of them. A game that emphasizes winning calculations from the wilds feature so that every image or symbol on the slot machine can become attached and count for points. Do not miss the opportunity to experience the highest Maxwin jackpot win by joining us on the best Gacor Maxwin slot gambling site, just register and use just a penny and you can play all the games straight away. Enjoy the newest type of online slot game that will be available in November 2023, there are profitable features that you will never find anywhere else.
Buy Tynee Mini 2 Electric Skateboard VS Exway Wave Electric Skateboard. Portable mini electric skateboards are very convenient for daily commuting. They are easy to carry and fun to ride. Among all the short electric skateboards. Tynee mini 2 and Exway wave are recognized as kings and loved by customers. Today, lets compare Tynee mini 2 and Exway wave in depth. Tynee mini 2 use famous Molicel 12S2P 363Wh battery. You can get up to 25 miles / 40 km range. While Exway wave use 10S2P 180Wh battery. You can get up to 12 miles / 20 km range. So you will get more range on Tynee mini 2. And Tynee mini 2 battery capacity is twice that of Exway wave. Which means you will get longer battery lifespan on Tynee mini 2. Because if you ride the board for 1000 km. On Tynee mini 2 you only need to charge and discharge the battery for 25 times (cycles). But on Exway wave you may need to charge it and discharge it for 50 times (cycles). So Tynees battery will decay slower and have a longer lifespan. As we all known, all the batteries will gradually decay over time. leading to a decrease in capacity. So we recommend customers buy a bigger battery for their board. For example. If you want 20 km range. if you get a small battery. You may feel the range is enough in the beginning. But after some time of using. You may find the range drop to 12 km and it doesn’t meet your requirement anymore. Then you have to buy a new battery and usually the battery is very expensive. So you will cost more. But if you buy a bigger battery in the beginning. For example you buy a board which can get 35-40 km range. Even after a long period of use. You may still get 30 km range. So it can still meet your requirement. So you don’t need to buy a new battery. You spend a little more in the beginning but save a lot in the future. And you don’t need to charge your board frequently. This will save a lot of time and be more convenient to use in daily life.
Which is the fastest way to lose weight? Netizens voted for "The 5 Most Effective Ways to Lose Weight" When it comes to the topic of weight loss, the editor is the first to sign up. I have missed many paths. At that time, I was very popular for a while. I just ate an apple every day and drank water, and I really lost 4 kilograms. Because its almost been a week, I always feel that I can not stand it, so I start to eat normally again. In just 4 days, I gained another 5 kilograms. Then there was a big problem: constipation started, and finally I had no choice but to go to the doctor. This bitter experience is still unforgettable to this day... Today I share with you five weight loss methods that are highly discussed on the Internet. Lets take a look. TOP1: "962 Diet", "66 Diet" Many people have only heard of "168" but not "962" or "66". In fact, this is the way for female celebrities to lose weight. 66 Diet This is a weight loss method that Tiffany Hsu used. She lost about ten kilograms just by using the "66 weight loss method". So what does this "66" mean? The first one is to only eat six percent full at each meal. Six percent full is actually more difficult for most people because it requires willpower to restrain; The second and last meal is finished at six o'clock in the evening, so it is called the "66 Diet". Generally speaking, if you are hungry, drink more water and black coffee. In addition, there are actually three things that need to be added to the "66 Diet" that cannot be touched.
UFABET login online football betting website. ufabet login in modern times Ufabet entrance It is considered important during safety in the UFABET entrance channel. and user friendliness Entrance to the UEFA website , which means responding to needs Online football betting by users in terms of technology Meanwhile It prevents access. without permission effectively There is a system that worksperfectly for live football betting. Take various measures Authentication with a face scanner to protect against threats. UEFA login There is security. ufabet login with the convenience of users The best football betting website It remains smooth and user-friendly. online casino Automatic data entry system Users no longer need to remember their passwords. ufabet Login makes the experience easy and adds another layer of security. By sending a one-time password to phone number registered user Football betting websites are designed meticulously To meet the needs of the era by prioritizing Both measures and experience that is user friendly effectively in our increasing reliance on Recognition and use of such systems More important in scope to protect personal information.
UFABET online gambling website, real payout 100% UFABET, the best direct website, gambling website, football betting website. UFABET, the main website for online gambling, which website is good? If you are asking for a direct website, not through an agent, which website is good? We are the best gambling website. There are many different services available which are reliable football betting websites that can bet on. UFABET online gambling There are many differences and diversity when talking about Online gambling website UFABET Nowadays, the service can be easily accessed and used by everyone. Of course, everyone must think of the UFABET website because it has the most complete and complete service and is. Online football betting website so that everyone can come and experience UFABET website There are many different types of betting games which are available for self-service and there is a lot of profit from betting which has a fully integrated service format and provides the best convenience. online gambling website that offers more services than various criteria which will be gathered together as UFABET casino to open the service for everyone to bet with Football betting website that pays for real And you can play for real money 100% for sure. Online football betting website UFABET It is a website that answers many gamblers' needs because it is UFABET is a direct website, not through an agent. Which can support every type of bet and is used in many languages and has developed and improved the website to be accessible on all platforms such as UFABET mobile, UFABETMOBILE, UFABETLOGIN, allowing everyone to easily become a member, which has many entrances that are a new dimension in Absolutely limitless betting to entertain everyone, including popular sports regarding football criteria with people around the world that everyone likes. Can apply for football betting with an online football website. Ours with safe and reliable use. With every bet, you will be able to receive long-term bets easily and we can guarantee that everyone who comes to use the service in the form of football betting will definitely not be disappointed.
ABB Temperature Transmitter The ABB TTF200 temperature transmitter is a universal input smart temperature transmitter. To adjust, it is designed to meet the specific application needs of measuring low-level signals from thermocouples (THC), resistance thermometers (RTD), resistance (ohm), or EMF (mV) sources. Aria Measuring Instruments Company is the sales representative of ABB temperature transmitters in Tehran and other cities. To buy eBay temperature transmitters or to get the catalog and price list of eBay temperature transmitters, contact our experts at Aria Measuring Instruments. ABB pressure transmitter has a range of modular electronics based on microprocessor and multiple sensor technology that performs pressure measurement with utmost precision in industries. You can use these tools and equipment for all your projects and processes in any condition, and you will definitely be satisfied with the result. Aria Measuring Instruments Company is the sales representative of ABB pressure transmitters in Tehran and other cities. To buy an eBay pressure transmitter or to get a catalog and price list of an eBay pressure transmitter, contact our experts at Aria Measuring Instruments. With a modular design, this product uses the latest advances in digital sensing technology, including a built-in smart chip and an interface to upgrade with new data collection methods such as NB IoT, offering the full benefits of digitization for better measurement and data sharing. The EBB level transmitter is the ideal solution for water and wastewater treatment applications, where dozens of level devices may be used, a product that offers simple setup, reliable performance, fast delivery and easy maintenance, provides tremendous value to the customer. Aria Measuring Instrument Company is the sales representative of ABB Level Transmitter in Tehran and other cities. To buy an eBay level transmitter or to get a catalog and price list of this product, contact our experts at Aria Measuring Instruments. With digital signal processing (DSP) and advanced filtering techniques, these innovative ABB flowmeters enable excellent flow signal detection and provide measurement safety against the effects of hydraulic noise and pipeline vibration. Aria Measuring Tools Company is the sales representative of ABB vortex flowmeter in Tehran and other cities. To buy an eBay flowmeter or to get a catalog and price list of this product, contact our experts at Aria Measuring Instruments. We have a complete portfolio of industrial technology products for customers in utilities, industry, transportation and infrastructure. We have a wide range of products, systems, services and solutions for customers in a large number of industries.
JOIN RICHES168 : Todays Gacor Slot Site RICHES168 Easy to Win Sensational Maxwin RICHES168 is a provider of viral online slot games in Thailand, our presence will certainly help you have an enjoyable playing experience. We have officially held a license to operate as the best Gacor slot site in Thailand and the distributor of the most popular RICHES168 provider. The RICHES168 gacor slot site offers the most complete collection of slot games, the highest RTP, ease of transactions, 24 hours customer service and much more. This offer certainly makes RICHES168 one of the targets of online slot fans in Thailand. 5 Gacor Slot Games that are Viral Today and Gacor Slot Leaks Looking for Gacor slots today is indeed one of the right steps for every slot player who wants to enjoy the Maxwin jackpot.
UFABET login online football betting website. ufabet login in modern times Ufabet entrance It is considered important during safety in the UFABET entrance channel. and user friendliness Entrance to the UEFA website , which means responding to needs Online football betting by users in terms of technology Meanwhile It prevents access. without permission effectively There is a system that worksperfectly for live football betting. Take various measures Authentication with a face scanner to protect against threats. UEFA login There is security. ufabet login with the convenience of users The best football betting website It remains smooth and user-friendly. online casino Automatic data entry system Users no longer need to remember their passwords. ufabet Login makes the experience easy and adds another layer of security. By sending a one-time password to phone number registered user Football betting websites are designed meticulously To meet the needs of the era by prioritizing Both measures and experience that is user friendly effectively in our increasing reliance on Recognition and use of such systems More important in scope to protect personal information.
UFABET is one of the betting circles and Online casinos are online gambling websites. that offers a wide variety of sports and casino games, from baccarat, roulette, slots, poker, and the lotto lottery. Meet a variety of gameplay. It gives you the opportunity to enjoy your favorite games. Moreover, UFABETWIN also has a Live Casino where players can get an authentic experience through live streaming video. Without having to travel to a real casino, so if you are looking for a diverse and exciting betting experience, Ufabet is the answer you are looking for. Why should you apply for UFABET, direct website, football betting website, online football betting with UFABETWINS website? UFABET direct website Not through an agent is a website that is suitable for users. that are diverse such as mobile phones and have various applications comfortable for iOS and Android The UFABETWINS website is an online gambling and casino website that offers many games such as baccarat, roulette, slots, poker, and the lotto lottery. And there are the best live casinos. Focus on making your online gambling comfortable and flexible. Deposits can be made easily and there are attractive promotions. UFABETWINS.ai It's the best choice. for online gambling There is convenience and variety in playing the game. And there are many interesting promotions.UFABET is one of the betting circles and Online casinos are online gambling websites. that offers a wide variety of sports and casino games, from baccarat, roulette, slots, poker, and the lotto lottery. Meet a variety of gameplay. It gives you the opportunity to enjoy your favorite games. Moreover, UFABETWIN also has a Live Casino where players can get an authentic experience through live streaming video. Without having to travel to a real casino, so if you are looking for a diverse and exciting betting experience, Ufabet is the answer you are looking for. Why should you apply for UFABET, direct website, football betting website, online football betting with UFABETWINS website? UFABET direct website Not through an agent is a website that is suitable for users. that are diverse such as mobile phones and have various applications comfortable for iOS and Android The UFABETWINS website is an online gambling and casino website that offers many games such as baccarat, roulette, slots, poker, and the lotto lottery. And there are the best live casinos. Focus on making your online gambling comfortable and flexible. Deposits can be made easily and there are attractive promotions. UFABETWINS.ai It's the best choice. for online gambling There is convenience and variety in playing the game. And there are many interesting promotions.
Welcome to Hall of Flame, your ultimate destination for top-tier cannabis experiences! As the premier cannabis dispensary in USA, we take pride in offering a diverse selection of premium strains, edibles, and accessories. Explore our curated collection, guided by knowledgeable staff passionate about enhancing your cannabis journey. Conveniently located in the heart of USA (delivery to All States), Hall of Flame stands as your go-to cannabis store, bringing you the finest products. Discover excellence in every visit at Hall of Flame – your trusted cannabis dispensary near me. Hall of Flame Discover a premier cannabis dispensary near you at Hallofflamez.net! Located in USA , we offer a curated selection of high-quality cannabis products to meet your unique preferences. Our knowledgeable staff is dedicated to providing a personalized and informative experience. Visit us for a diverse range of strains, edibles, and accessories. Medical cannabis can be administered through various methods, including capsules, lozenges, tinctures, dermal patches, oral or dermal sprays, cannabis edibles, and vaporizing or smoking dried buds. Synthetic cannabinoids are available for prescription use in some countries, such as dronabinol and nabilone. Countries that allow medical use Explore the world of premium cannabis at Hallofflamez.net – your trusted local destination.
Gacor IDNPoker Online Slot Bookie, Best Sportbook, Casino Traditionbet is present as the best online gambling site in Indonesia with a large selection of games that offer various promotional programs and interesting events. Traditionbet is a pioneer site that presents many types of games in 1 (ONE) account such as Online Slots, Esports, Idnlive, Togel, Live Casino, Mickey Mouse, Tangkas, Sportbook, and many other exciting games. Traditionbet presents an exciting gaming experience with a modern graphic display that will add to the exciting sensation of the game, the latest and leading technology is carried out by a team of experts and professionals in their field which will ensure the game is safer and more comfortable. Every transaction process at Traditionbet is faster because it is supported by many choices of the best private banks as well as various choices of popular payment methods. It is very important if you play online slots on an online gambling site whose credibility is guaranteed, for example an official online gambling site like TRADISIBET. With TRADISIBET, the leading online gambling site, all your worries will definitely disappear. Any betting will definitely run smoothly and more importantly the winnings will be 100% guaranteed to be paid regardless of the nominal amount.
Introduction to the 1xBet Promo Code Looking to get ahead in the world of sports betting? The 1xBet promo code is: TURBOGOLD your ticket to a lucrative cash match bonus of up to €/$130. With a wide range of sporting events and markets on offer, 1xBet is an exciting platform that offers plenty of opportunities to win big. Whether you are new to online sports betting or a seasoned pro, the 1xBet promo code TURBOGOLD provides a fantastic way to boost your account balance and increase your chances of success. Lets take a closer look at how it works. Current promo codes for 1xBet ✓ Promotion 2024 ✓ Bonus codes ✓ Registration bonuses ✓ Loyalty Program ✓ Cashback bonus! 1xBet Promo code : Bonus1x200 - this combination gives you an exclusive sports bonus that will add 100% to your first deposit of up to 130 €/$. To receive bonuses, the client of the 1xBet bookmaker needs to fulfill certain requirements, then promo codes can be obtained much easier. Naturally, the amount of bonuses that become available to players through 1xBet promo codes is small. Nevertheless, even a small bonus can significantly increase the clients gaming potential.
22Bet Promo Code : MAX200 • Bonus 100% up to €122 - 22Bet promo code: MAX200. All new players take part in the 2024 Promotion and receive a 100% welcome bonus up to €122. The bonus code is used during registration. 22Bet offers an active bonus campaign for new users, you can read more in our review. This is available at all locations using this franchise. Moreover, each subsidiary company can adjust all shares according to its wishes and preferences. the 22Bet promo code MAX200 provides a fantastic way to boost your account balance and increase your chances of success. Lets take a closer look at how it works. Current promo codes for 22Bet ✓ Promotion 2024 ✓ Bonus codes ✓ Registration bonuses ✓ Loyalty Program ✓ Cashback bonus! 22Bet Promo Code : MAX200 - this combination gives you an exclusive sports bonus that will add 100% to your first deposit of up to 122 €/$. To receive bonuses, the client of the 22Bet bookmaker needs to fulfill certain requirements, then promo codes can be obtained much easier. Naturally, the amount of bonuses that become available to players through 22Bet promo codes is small. Nevertheless, even a small bonus can significantly increase the clients gaming potential.
Welcome to the party of my life here you will learn everything about me. I also wrote an article on a similar subject will find it at writing what you think. Very good topic, similar texts are I do not know if they are as good as your work out.
ORI99: Trusted Official Place to Play Online Games 2024 Ori99 is a trusted official online gaming site in 2024 and is suitable for those of you who are looking for the best gaming sensation without getting bored. Not only that, playing on ori99 is quite easy, all you need to do is register an account in less than 2 minutes and you can play hundreds of thousands of games. Talking about online gaming sites, Ori99 login is a solution for you, because only here you can get big promotions and bonuses that won't make you lose money, especially Ori99 has various overseas servers such as Thailand, Vietnam, Malaysia, Cambodia, Japan, and China. What are you waiting for, register your account immediately without any hassle or conditions.
Gacor Slot: Pragmatic218 Trusted Online Dewa Slot88 Site 2024 Welcome to the Pragmatic218 Gacor site, its easy to win, here we provide anti-gloomy Gacor slot games such as Pragmatic Play, Slot88, Habanero, Spadegaming and Microgaming. Pragmatic 218 as a gacor site also provides accurate Live RTP Slots for novice members. We are also an official pragmatic play agent who can make the dreams of thousands of members who are looking for high income come true. For this reason, do not hesitate to register for the gacor pragmatic218 feat dewaslot88 antisuram slot right now!! The Most Accurate Live RTP Slot Game in the Year of the Dragon Several criteria for live RTP slot games that you must play: 1) Gacor Gate Of Olympus Slot Game. 2) Gacor Zeus Slot Game. 3) Gacor Mahjong Ways Slot Game. 4) Gacor Koi Gate Slot Game. 5) Gacor Sweet Bonanza Slot Game. 6) Gacor Gatot Kaca Slot Game. 7) Gacor 5 Lions Slot Game. 8) Gacor Hot Hot Fruit Slot Game. So what are you waiting for, come and play with the god slot88 gacor Pragmatic218 site, if you have not played yet? Its a loss donggg..
Unveiling the Livetotobet Totobet Server: Pioneering International Gambling Since 2010. Livetotobet and totobet became the first online game providers to use international servers and collaborate with foreign providers for the advancement of gaming technology. Unveiling the Livetotobet Totobet Server Gambling has come a long way from dimly lit back rooms to the vibrant and dynamic online landscape we know today. In this ever-evolving sphere, one name has stood the test of time and continually pushed boundaries –Livetotobet Totobet Server. Established in 2010, it has not just weathered the storms of the digital age but has emerged as a pioneering force in the international gambling scene. Imagine a world where the thrill of placing Livetotobetand the suspense of a live draw converge seamlessly into a digital experience. Thats precisely what Livetotobet Totobet Serverbrought to the table. From its inception, it was not merely a gambling platform; it was a vision to redefine the gambling experience for enthusiasts around the globe.Breaking Grounds Beyond BordersIn the vast universe of online gambling, where competition is fierce and innovation is the currency, Livetotobet Totobet Servertook a bold step by going beyond domestic boundaries. This server, with its international outlook, ensured that players worldwide could partake in the exhilarating journey it offered.The Totobet Experience: What Sets it Apart? In a market flooded with options, Livetotobet Totobet Serverstands out as a beacon of reliability, excitement, and, most importantly, trust. Lets dive into what makes this gambling server a trailblazer: 1. Seamless User InterfaceNavigating through the platform is as smooth as a hot knife through butter. The user interface is designed with simplicity in mind, ensuring that even beginners can comfortably find their way around. Bold icons and intuitive menuscreate an immersive experience, leaving players focused on the thrill rather than grappling with complexities. 2. Variety of GamesBoredom is not in the vocabulary of Livetotobet Totobet Server. The platform boasts an extensive array of games, catering to diverse tastes and preferences. Whether you are a fan of classic casino games or crave the excitement of live sports betting, Totobet has something for everyone. Its like having a buffet of entertainment options at your fingertips.
Order Claude Monet poster As one of the founders of French Impressionism, Oscar-Claude Monet (1840–1926) broke away from the convention of copying the Old Masters. Monets Impressionist artworks depict his direct observation of nature and emphasis on shifting lights according to different times and seasons. This vintage illustration collection features Monets beautiful landscape and portrait paintings, including his famous Water Lilies, Haystacks and Rouen Cathedral. Claude Monet is best known for his landscapes and cityscapes in the Impressionist style. He often painted in the open air, trying to convey the direct impression of light and color on his subject. Monet became famous for his beautiful paintings of nature, including his best-known works Series of Water Lilies and Impression, Sunrise. He is often seen as one of the most important representatives of the Impressionist style and as “the father of landscape. All photographs on this website, including images of artwork, are royalty-free and used in accordance with applicable licenses or with permission of the owner. Grootmeesterkunst.nl strives to only use photos for which appropriate permission has been obtained and that do not infringe the intellectual property rights of others. Affordable wall art Claude Monet At GrootmeesterKunst.nl you can order affordable posters of well-known artists. A Claude Monet poster is available in A4, A3 and A2 format. You can easily order a poster slat or frame with the poster. With us you will find a huge range of unique and affordable art. Claude Monet poster + frame You can easily order a Claude Monet poster with an aluminum or wooden frame from Grootmeester Kunst.nl. Combine several posters in your home or bedroom as affordable eye-catchers for your interior. Order 2 posters and also receive a 20% discount. You can keep one for yourself or give it away as a gift. A classic poster to decorate your home. Prints by Claude Monet Claude Monet prints are of high quality at Grandmaster Art. Order your poster today before 4:00 PM and receive the print tomorrow. Do you have any questions or would you like to know more about our website? Please feel free to contact us.
New gambling website The one that is giving away the most is Space 789. Space 789 is a hot new gambling website that has just been launched recently. But it quickly gained popularity. With promotions and bonuses that are many and worthwhile. causing gamblers to flock together to apply for membership in droves As for the promotions at the entrance to Space 789 , it can be said that they are quite intense. Whether its a promotion, apply for new Space 789 and receive a 100% bonus, a first deposit promotion and receive a 50% bonus, a promotion to invite friends to receive a 1% bonus, a promotion to return every balance 3%. and many more. There are also many events and tournaments held on a regular basis. So that gamblers can join in the fun and win big prizes. In addition to many promotions, the Space 789 website also has many types of gambling games to choose from, such as baccarat, slots, roulette, dice, football betting, lottery betting, and many more. Each game is a game. It is popular with gamblers all over the world. As for the system of Space 789, the direct website is designed to be easy to use. Supports both Thai and English languages. Deposit and withdraw money quickly Safe with a modern security system. With many and worthwhile promotions Easy to use system Various gambling games And reliable security makes the website to play Space 789 become a new gambling website that is hot and quickly gaining popularity. Play slots at Space 789, the biggest prizes are the easiest to win. For anyone who likes playing online slot games. Do not miss out on space 789 slots because there are many slot games to choose from. Whether its slot games from famous camps like PG Slot, Joker Slot, JDB Slot, and many others, each game is a quality game, easy to play, easy to win, lots of big prizes. In addition, Space 789 also has promotions and Huge bonuses for slot games as well. Whether its a promotion for new members to receive a 100% bonus, return up to 3% on every balance, invite to apply to get 1% on every friends playing balance, and 100 free spins, it can be said to be worth it. Definitely an investment.
MAXWIN66: Todays Online Gacor Slot Site, Easy MaxWin. MAXWIN66 is the most popular Gacor slot site, Maxwin which is also known as one of the best slot gambling sites providing the most complete slot games officially licensed by Pagor, Maxwin66 slots collaborates with many providers to create viral slot games which are definitely Gacor according to our name Maxwin66 with The win rate is 97% so you can feel the exciting sensation of playing slots on the Maxwin66 site with maximum wins. Playing at MAXWIN SLOT is the right thing because you Gacor online slot lovers of course want to experience playing slots with maximum wins, Maxwin66 is known as one of the Gacor slot gambling sites that you can trust and of course Maxwins victory is in sight, we provides the latest slot games which are guaranteed to have a guaranteed level of success, register at maxwin66 and try to feel the maximum sensation of winning playing slots with us.
I should assert barely that it's astounding! The blog is informational and also always fabricates amazing entities. Acknowledges for penmanship such a worthy column, I stumbled beside your blog besides predict a handful of advice. I want your tone of the manuscript...
We Provide Business Cards which shows your whole profile within one tap on any phone. which means we replace traditional paper visiting cards to a single PVC Digital Business Card. Revolutionize your Visiting Card with Artificial Intelligence We have Developed AI Based NFC Visiting Cards, which will show your Complete Business/Profession or Personal Details with just a Touch on your Cellphone. We Offer a Wide Range of Digital NFC Cards for Your Resume and for your Business Details. In todays rapidly evolving digital age, businesses are constantly seeking innovative ways to connect with potential clients, partners, and customers. One such revolutionary technology that is reshaping the way we exchange information is Near Field Communication (NFC). With its seamless data transfer capabilities, NFC has found a new application in the world of business cards, replacing traditional paper cards with smart, interactive alternatives. We are trusted by over 5000+ clients. Join them by using our services and grow your business.
Amazing, this is great as you want to learn more, I invite you to This page. Your texts on this subject are correct, see how I wrote this site is very good. For true fans of this thread, I will address it free online! Can nicely write on similar topics! Welcome here you'll find out how it should look.
Asbola The Safest and Most Trusted Football Betting Site. Asbola is the most trusted and safest soccer betting site in Indonesia today. Our official soccer betting site is the most popular among people in Indonesia. For Indonesian people who are still looking for a place to play soccer betting. We recommend that you register with Asbola because our site is always the first choice for big players in Indonesia. For this reason, you players must move quickly and register immediately on our site. As a trusted soccer betting site, Login Asbola . Provides various kinds of sportbox games. Such as mix parlay, handicap. Over/under, correct score, and many other games that you can play on our trusted site. and we will always provide comfort and security for the members who play here. Asbola is a soccer betting site that has one of the most complete soccer betting markets among other soccer betting sites. Therefore, players from Indonesia always look for our site to play here. One thing we emphasize is that for the members who play here, we will really guarantee your safety and there will be no problems whatsoever. We have also prepared an alternative Asbola link so that it is easier for you to access and log in to our website.
Slot Online Terbaik Gampang Turun Perkalian dan Mudah Jackpot. Cemara777 telah menjadi pilihan utama bagi para pecinta judi slot online di Indonesia. Dikenal sebagai platform terbaik, Cemara777 menawarkan pengalaman bermain yang memikat dengan beragam permainan slot yang menarik. Salah satu keunggulan utama dari Cemara777 adalah ketersediaan link alternatif yang selalu terupdate, memastikan akses yang lancar tanpa hambatan.
Amazing, this is great as you want to learn more, I invite you to This page. Your texts on this subject are correct, see how I wrote this site is very good. For true fans of this thread, I will address it free online! Can nicely write on similar topics! Welcome here you'll find out how it should look.
I invite you to the page where you can read interesting information on similar topics. I have a similar interest this is my page read everything carefully and let me know what you think. Interestingly you write, I will address what you'll find exciting and interesting things on similar topics. Cool you inscribe, the info is salubrious and further fascinating, I'll give you a connect to my scene.
kurir69 : Todays Trusted Gacor Slot Gambling Site. Are you looking for gacor slots today? If it is true then kurir69 is the right answer. As an easy-to-win Gacor slot gambling site, we have various trusted online gambling games licensed for circulation. Without having to wait any longer, here is todays gacor game with maxwin wins and you can only get all of it on the trusted online slot gambling site kurir69. kurir69 is committed to legalizing responsible betting as well as promoting awareness of problem gambling and improving prevention, intervention and services. kurir69 Gaming Responsibility Policy sets out its commitment to minimize the negative effects of problem gambling and to promote responsible gambling practices. We believe it is our responsibility to you, our customer, to ensure that you enjoy your betting experience on our site, while remaining fully aware of the social and financial harm associated with problem gambling. In order to assist our players in responsible gambling, we ensure that all our staff have responsible gambling awareness. Please contact us if you need further information or assistance.
PTBOLA EURO 2024 PARLAY PARLAY GAMBLING AGENT. PTbola is a soccer parlay betting place and a partner for the Euro Cup 2024, the best and most trusted online gambling service at the moment, being the best site is certainly not an easy thing for all gambling sites to achieve, and currently there are many who want to stand up to become an online gambling site but most of them do not have good rules because there are sites that are not guaranteed to be safe for players to win. Of course, with the PTbola gambling site, now you do not need to worry about parlay soccer betting, online slot gambling, casino gambling, aduqq gambling or other gambling, because PTbola has been recognized by the entire community that PTbola is one of the biggest agents that can pay all players winnings. without any further ado. By having an account with an official football agent, of course PTbola is also very practical to access wherever you are. By accessing the PTbola gambling site, you can play all the games using just 1 user ID. Depositing with the PTbola site now does not require large capital, with just 25 thousand capital you can play. So, to join the PTbola site, do not worry, of course you will also get a bonus for players who have just joined. With a minimum deposit of 100 thousand, you can get 10% of that deposit. Providing bonuses for new users is also something that is very rarely done by any football agent, so do not make the mistake of choosing the trusted football betting site PTbola.
New gambling website The one that is giving away the most is Hokiemas88. Hokiemas88 is a hot new gambling website that has just been launched recently. But it quickly gained popularity. With promotions and bonuses that are many and worthwhile. causing gamblers to flock together to apply for membership in droves As for the promotions at the entrance to Hokiemas88, it can be said that they are quite intense. Whether its a promotion, apply for new Hokiemas88 and receive a 100% bonus, a first deposit promotion and receive a 50% bonus, a promotion to invite friends to receive a 1% bonus, a promotion to return every balance 3%. and many more. There are also many events and tournaments held on a regular basis. So that gamblers can join in the fun and win big prizes. In addition to many promotions, the Hokiemas88 website also has many types of gambling games to choose from, such as baccarat, slots, roulette, dice, football betting, lottery betting, and many more. Each game is a game. It is popular with gamblers all over the world. As for the system of Hokiemas88, the direct website is designed to be easy to use. Supports both indonesian and English languages. Deposit and withdraw money quickly Safe with a modern security system. With many and worthwhile promotions Easy to use system Various gambling games And reliable security makes the website to play Hokiemas88 become a new gambling website that is hot and quickly gaining popularity. Play slots at Hokiemas88, the biggest prizes are the easiest to win. For anyone who likes playing online slot games. Do not miss out on Hokiemas88 slots because there are many slot games to choose from. Whether its slot games from famous camps like PG Slot, Joker Slot, JDB Slot, and many others, each game is a quality game, easy to play, easy to win, lots of big prizes. In addition, Hokiemas88 also has promotions and Huge bonuses for slot games as well. Whether its a promotion for new members to receive a 100% bonus, return up to 3% on every balance, invite to apply to get 1% on every friends playing balance, and 100 free spins, it can be said to be worth it. Definitely an investment.
Gacor Slot: Hokiemas88 Trusted Online Dewa Slot88 Site 2024 Welcome to the Hokiemas88 Gacor site, its easy to win, here we provide anti-gloomy Gacor slot games such as Pragmatic Play, Slot88, Habanero, Spadegaming and Microgaming. Hokiemas88 as a gacor site also provides accurate Live RTP Slots for novice members. We are also an official pragmatic play agent who can make the dreams of thousands of members who are looking for high income come true. For this reason, do not hesitate to register for the gacor Hokiemas88 feat dewaslot88 antisuram slot right now!! The Most Accurate Live RTP Slot Game in the Year of the Dragon Several criteria for live RTP slot games that you must play: 1) Gacor Gate Of Olympus Slot Game. 2) Gacor Zeus Slot Game. 3) Gacor Mahjong Ways Slot Game. 4) Gacor Koi Gate Slot Game. 5) Gacor Sweet Bonanza Slot Game. 6) Gacor Gatot Kaca Slot Game. 7) Gacor 5 Lions Slot Game. 8) Gacor Hot Hot Fruit Slot Game. So what are you waiting for, come and play with the god slot88 gacor Hokiemas88 site, if you have not played yet? Its a loss donggg..
Panenslot | Super Gacor Online Slot Game Site Definitely Maxwin 2024. Are you looking for gacor slots today? If it is true then Panenslot is the right answer. As an easy-to-win Gacor slot gambling site, we have various trusted online slot88 gambling games licensed for circulation. Without having to wait any longer, here is todays gacor slot88 game with maxwin wins and you can only get all of it on the trusted online slot gambling site Panenslot. Panenslot is committed to legalizing responsible betting as well as promoting awareness of problem gambling and improving prevention, intervention and services. Panenslot Gaming Responsibility Policy sets out its commitment to minimize the negative effects of problem gambling and to promote responsible gambling practices. We believe it is our responsibility to you, our customer, to ensure that you enjoy your betting experience on our site, while remaining fully aware of the social and financial harm associated with problem gambling. In order to assist our players in responsible gambling, we ensure that all our staff have responsible gambling awareness. Please contact us if you need further information or assistance.
KUYBET merupakan Situs Judi Casino Online, Slot Gacor Resmi Dan Berlisensi. Menyediakan Berbagai Permainan Casino Online Terbaik Serta Berbagai Pilihan Permainan Slot Gacor Cukup Dengan Deposit 10 Rb Anda Sudah Bisa Bermain Seluruh Permainan Kami. Katakan Tidak Pada Rungkad. Temukan Maxwin Hanya Di KUYBET Situs Slot Gacor Hari Ini.
Within this webpage, you'll see the page, you need to understand this data. It is quite beneficial, although think about the facts when it reaches this target. I might suggest solely beneficial in addition to trusted facts, and so find it:
ONICTOTO: The Biggest Official Online Bookie in Asia. ONICTOTO provides the latest online games with online access plus extensive device compatibility support. You have no obligation or obligation to always play from a computer or laptop. If you are more comfortable gambling online from a smartphone or tablet, this can be done. The type of device is not regulated at all as long as internet access is available and there is a browser to use to open the ONICTOTO site. In fact, you do not need a special browser and just use the one that is already installed. The operating system is also not a limitation. Whether its Windows, Android, or iOS, everything will work. Players who are trying the game for the first time on the ONICTOTO site may still feel confused and not yet used to the access provided. However, this is not a problem at all. There is customer service support that will be ready to help gamblers. In fact, the customer service team is always active 24 hours so you can always ask questions at any time. To make things easier for you and other bettors, there is live chat access so you do not need to open a messaging application or anything else. Everything is available and ready to use.
OFFICIAL AND EASY TO ACCESS UG555 2024 GAMES WEBSITE. UG555 is an official link that is easy to access and trusted with a dynamic & modern appearance. Login & Register now at the alternative link UG555. As a solution for you online game lovers, we are here with easy access, UG555 can be played anywhere & anytime and can be downloaded via your favorite gadget. We also provide the biggest online games in the world. Apart from that, UG 555 will definitely give you comfort in playing and especially easy access to the official UG555 2024 link.
Why Choose Byfavorites Cart? There are several key reasons Byfavorites carts stand out from the competition: High-Quality Ingredients: All Byfavorites vape products contain at least 95% hemp-derived Delta-8 THC oil and premium-grade terpenes. They use no cutting agents or other fillers that can degrade quality and potency. Third-Party Lab Tested: Every batch of every Byfavorites cart flavor and strain undergoes independent third-party testing at ISO-certified labs. This ensures safety, purity, potency, and compliance. Great Flavor Variety: Shoppers can choose from tantalizing flavors like Gelato, Zkittlez, Strawberry Cough, and Sour Diesel, along with effects-based options like Calm, Sleep, and Focus.
Buy Instagram Followers: Unveiling the Excellence of TopFollowers.co In the fast-paced world of social media, buying Instagram followers can be a game-changer for both individuals and businesses. The appeal of a significant following is undeniable and contributes significantly to brand credibility and online visibility. In recent times, the concept of buying Instagram followers has been gaining momentum, with platforms like TopFollowers.co emerging as the go-to solution for those looking to increase their follower numbers. Why Instagram followers are important The digital landscape is crowded and standing out requires more than just compelling content. A high follower count acts as a virtual endorsement and signals to others that your content is worth following. Brands with a substantial following are more likely to attract new audiences, and individuals can use a large following to increase their influence. The rise of TopFollowers.co In this scenario, TopFollowers.co has come to the fore as a reliable and reputable platform for buying Instagram followers. With a track record of providing quality services, this platform offers a range of benefits that set it apart from the competition. Quality of followers One of the main factors that sets TopFollowers.co apart is the quality of followers it offers. Unlike some services that offer bot accounts, TopFollowers.co ensures that every follower is genuine and engaged. This commitment to quality ensures that the followers you gain contribute to your online presence in a meaningful way.
In the case of the companies recommended by Poker Bud, which are licensed Evolution Casinos where KRW can be deposited, Evolution Casino & Microgames are legally certified for casino licenses and pay taxes and license fees, so it is impossible to scam them. Depending on each site, online casino games include Evolution Casino, Microgame (Microgaming), Taishan Casino, Allbet, Asian Gaming (Asian Games), and Oriental Casino games. Additionally, we are partners with major overseas sports betting sites: Sbobet, MaxBet, and Pinnacle Sports. Now there is no need to use a private Toto site. This is because you can rest assured through a Korean-style overseas betting site that allows you to deposit KRW right away!
Todays Latest Gacor Maxwin 777 Online Slot Link 2024 - RAFIGAMING. The newest slot link from slot 777 on rafigaming, Slot 777 Gacor today and Maxwin anti rungkad is not just a figment of the imagination. Because Rafigaming is proven to often provide anti-crash slot game patterns to all members. Slot Gacor Anti Rangkad - RAFIGAMING The Rafigaming site has started to be in the spotlight for slot members since the viral slogan for the gacor slot anti rungkad went viral. Every member definitely really wants to win Maxwin, right, so the right solution is to play at Rafigaming. Latest Gacor Slot Link - RAFIGAMING The latest gacor slot link can be registered through the rafigaming slot vip account where the site already has gacor patterns and accurate RTP for slot participants who like to play. Coin capital slots become sultan if you play on Rafigamings gacor anti rungkad slot site.
Iconwin is the Gacor Slot Site, the Most Complete Slot777 Game Provider in Indonesia. As a GACOR SLOT SITE that provides interesting games, ICONWIN will definitely always provide gacor benefits for all online game players in Indonesia. One of them is Online Slots which are most popular with all groups, here you will experience fantastic wins that you have never had before The Iconwin site uses the best and most trusted servers whose quality has been tested and has an official credit deposit slot license to guarantee members smooth and reliable playing of Indonesian online slots with real money. With the keyword SLOT777 which is popular in Indonesia, you will definitely experience maximum wins every day if you play with Iconwin. We always offer attractive promotions and bonuses for new players and loyal IconWin players. Such as daily bonuses, weekly bonuses, monthly bonuses, to annual bonuses! There are also incredible bonus promos from 50% to 200%!! Do not wait any longer, register immediately and become a champion with Iconwin, the Gacor Slot Site!
Introduction to Mega888 Apk Update Mega888 is a premier online gaming platform that has carved a niche for itself in the digital casino world. Known for its wide variety of games, it offers fans a virtual passage into a realm where fun and entertainment are unlimited. From classic slots to live table games, Mega888 apk caters to a diverse audience, ensuring there is something for everyone. Quick Overview of Mega888 Apk At its core, Mega888 malaysia update is designed with user experience in mind. The platform has a user-friendly interface, making navigation smooth for experienced players and newcomers alike. With a focus on reliability and fairness, it uses advanced security measures to protect user data and transactions, providing a safe and reliable environment for online gaming. Its popularity in the Online Casino Community Mega888 apks rise to fame can be attributed to its incredible quality and variety of games. Players are attracted by its vibrant graphics, exciting gameplay and the chance to win big. The platform commitment to regularly updating its game library keeps the experience fresh and engaging. Furthermore, its accessibility across multiple devices has broadened its appeal, allowing players to play their favorite games anytime, anywhere. What is Mega888? Mega888 is an online casino platform known for its wide variety of slot and table games. It offers a user-friendly interface, high-quality graphics and a safe gaming environment, making it popular among online casino enthusiasts in Southeast Asia. Is Mega888 safe to use? Yes, Mega888 uses advanced security measures, including encryption technology, to protect user data and transactions. It is also constantly audited for fairness and uses a certified random number generator for its games.
Rafigaming merupakan situs permainan taruhan bola online terbaik dan teraman di seluruh Indonesia tahun 2024.
2024 Snicasino is starting to become popular with the slogan of registering for the gacor online slot site today, it is definitely anti-cheat and can achieve maxwin wins. Snicasino is the best gacor slot site in 2024 with the latest slot link feature which will definitely provide an exciting experience for those of you who are traumatized by losing in slot games. Apart from that, apart from being trusted, the Maxwin Snicasino Slot also really protects the data privacy of each of our Gacor slot members. Todays term for gacor slots at Snicasino is super fantastic member wins of up to many times, even tens of times. Because we have applied the Accurate Slot RTP feature to each member so that we analyze first which slot providers have a NA percentage above 90 percent. In addition to Fairplay, the maxwin SNI Casino slot site even dares to give a whopping 100% guarantee to every slot player so that the Snicasino site became famous as an anti-rungkad slot.
Vibet77 is a thrilling online platform that has emerged as a sensation for entertainment seekers. Its impressive repertoire of games and dedication to fair play make it a must-try for all gaming enthusiasts. Unlike other online casinos that encourage players to cheat and hack their way ahead in games, Vibet77 promotes fair play and makes every gaming session on the site an enjoyable one. The platform is a safe haven for gamers and has a team of security experts that monitor the site around the clock to ensure all personal information and transactions are safe. It also uses advanced encryption modern technology to safeguard the data and privacy of its members. Furthermore, the site is committed to responsible pc gaming and offers players tools and resources that help them manage their gaming practices. Despite being a relatively new entrant in the online casino industry, vibet77 has made waves with its innovative technology and commitment to player safety and security. This has helped it stand out from its competitors and is poised to revolutionize the on the internet casino market. Moreover, the site is completely legal and has been verified by the relevant authorities to ensure that its operations are within the limits of regulations. In addition, its virtual reality innovation provides a unique experience that sets it apart from other online casinos. It also helps players get the feel of a real-life casino, complete with high-quality graphics and realistic sound effects. The app is also easy to use and requires no installation or download to function properly.
Todays Latest Gacor Maxwin 777 Online Slot Link 2024 - RAFIGAMING. The newest slot link from slot777 on rafigaming, Slot 777 Gacor today and Maxwin anti rungkad is not just a figment of the imagination. Because Rafigaming is proven to often provide anti-crash slot game patterns to all members. Slot Gacor Anti Rangkad - RAFIGAMING The Rafigaming site has started to be in the spotlight for slot members since the viral slogan for the gacor slot anti rungkad went viral. Every member definitely really wants to win Maxwin, right, so the right solution is to play at Rafigaming. Latest Gacor Slot Link - RAFIGAMING The latest gacor slot link can be registered through the rafigaming slot vip account where the site already has gacor patterns and accurate RTP for slot participants who like to play. Coin capital slots become sultan if you play on Rafigamings gacor anti rungkad slot site.
Buy Cialis without a prescription The health department seizes many fake aphrodisiacs every year . Most of these fake drugs contain local anesthetics, which can delay male sensitivity and make erections last longer. However, long-term use may cause serious harm to the body. These fake drugs are marketed as not requiring a prescription. No., are circulating in the market in large numbers. So how do we buy Cialis without a prescription? You can go to Cialiseshop.com, which is operated by Taiwan Pharmacy. Cialis is a prescription drug, In addition to changing lifestyle and dietary habits, medications are the main treatment for impotence. Currently licensed prescription drugs for treating impotence include Viagra, Cialis, and Levitra. All three drugs are approved by the U.S. Drug and Food Administration. Although these doctor-prescribed medications are generally safe, they cannot be sold in pharmacies. Viagra was the first drug approved for use by U.S. health agencies, but Cialis has become widely popular because it produces results more quickly. Cialis remains effective longer than other drugs and can remain active in the body for 36 hours, so it can provide couples with a more perfect sexual experience. And the effect can be produced 30 minutes after taking Cialis. Cialis is available in 10 mg and 20 mg tablets and can be taken orally before sex. At present, updated products have been launched, the dosage is 2.5mg and 5mg, it is recommended to take it daily. This drug is only effective when you are sexually aroused.
Indulge in Ultimate Relaxation: Discover Taipei Mens Stress Relief Club In the bustling streets of Taipei, amidst the hustle and bustle of everyday life, lies a sanctuary dedicated to the well-being of men – the Taipei Mens Massage and Stress Relief Club. Here, amidst the citys vibrant energy, men can find solace, relaxation, and rejuvenation like never before. Why Choose Us? At Taipei Mens Massage and Stress Relief Club, we prioritize your comfort, discretion, and satisfaction above all else. Heres why we stand out among the plethora of stress relief clubs in Taipei. The Stress Relief Experience: Our Stress Relief Red Card Popular Picks offer a glimpse into the luxurious experience that awaits you at our club. But experiencing it firsthand is where the true magic lies. Heres how it works: Consultation: Our customer service team is here to guide you through the process, discussing service options, locations, prices, and understanding your preferences. Selection: Choose from our carefully curated selection of stress relief options, tailored to meet your specific needs and desires. Arrival: As you step into our club, be greeted by our friendly staff, ready to assist you in making your experience unforgettable. Confirmation: Once you have made your selection, confirm your choice and prepare to embark on a journey of relaxation and indulgence. Stress Relief Begins: From here on, surrender yourself to the expert hands of our skilled aromatherapists, and let the stress melt away. Indulge in the Ultimate Relaxation Experience: Discover the oasis of tranquility that awaits you at Taipei Mens Massage and Stress Relief Club. Whether you are seeking relief from the stresses of daily life or simply craving a moment of pure relaxation, we invite you to experience the difference for yourself. Unwind, rejuvenate, and emerge feeling refreshed and revitalized – because you deserve nothing less.
Rediscover Intimacy with Viagra: The Key to Revitalizing Relationships. Are you or someone you know struggling with erectile dysfunction (ED)? It's a common issue that affects millions of men worldwide, often leading to frustration, anxiety, and strained relationships. But there is hope – and it comes in the form of Viagra. With over 130 million success stories, Viagra has been a game-changer for men seeking to reclaim their sexual happiness. Here is why: Regain Confidence: Viagra enhances hardness like never before, ensuring a firm and reliable erection that boosts confidence in the bedroom. Lasting Endurance: Say goodbye to performance worries with Viagra is ability to provide up to four hours of endurance, allowing for multiple satisfying encounters. Consistent Strength: With Viagra, there is no need to worry about deflation mid-act. It keeps you strong and erect throughout, ensuring a pleasurable experience for both partners. Safety First: Rest easy knowing that Viagra is backed by the U.S. FDA for its safety and effectiveness, making it a trusted choice in ED treatment. Versatile Solutions: Beyond ED, Viagra also helps prevent altitude sickness and treats prostate hypertrophy, showcasing its wide-ranging benefits. But what sets Viagra apart from other options? It is not just about the results; it is about the journey to achieving them. Here is what you need to know: Fast-Acting Relief: Viagra kicks in within 30 to 60 minutes, providing timely support when you need it most. Personalized Dosage: Tailor your dosage based on your needs, with options ranging from 25mg to 100mg, ensuring optimal efficacy and minimal side effects. Proven Success: Studies show that consistent use leads to increased success rates, with most experiencing peak results by the eighth use. Expert Guidance: Always follow your doctors recommendations for the best outcomes, and remember to take Viagra on an empty stomach for optimal absorption. Concerned about side effects? Do not be. While mild side effects like headache and flushing may occur, they are typically transient and manageable. Plus, with proper usage, the likelihood of experiencing adverse reactions is minimal. Ready to take the first step toward a happier, more fulfilling sex life? Look no further than Viagra. And with discreet packaging and fast, confidential delivery, you can purchase with confidence, knowing that your privacy is protected every step of the way. Do not let ED stand in the way of your intimacy. Choose Viagra and rediscover the joy of meaningful connections today. Buy Now and embark on a journey to rekindled passion and intimacy.
Acknowledges for paper such a beneficial composition, I stumbled beside your blog besides deciphering a limited announce. I want your technique of inscription...
Panen138: Gacor Online Slot Site Flooded with the Biggest Maxwin Jackpot. The Panen138 online slot site is the best choice for playing gacor slot games which are being sought by many slot lovers, because of the ease of registering and the ease of winning online slot gambling. With the Panen138 online slot site, you can choose a game according to your favorites. We also collaborate and have the best slot provider partners. This has been proven because every member provides testimonials after playing online slot gambling. The game has been developed with todays latest gacor slot update and is designed using the latest technology so that members can immediately play it online with very high loading speeds. Todays gacor slot game which is available at Agen Panen138 can be very popular because every player can win the maximum jackpot win 777 slot. For those of you who have just joined and played this game, you do not need to worry, we provide the right ways and steps to play online slots. We also have customer service serving 24 hours non-stop who is ready to help you at any time. Gacor slot leak information or in standard language as a grid for easy-to-win online slot games is presented here. Collection of the Best Online Slot Providers & Gacor Slot Games Today, Easy to Win. Currently there are many lists of the best online slot gambling sites, Panen138 is one of the officially trusted gacor slot sites today . However, we will provide some of the easiest slot providers to win, including the following: 1. Pragmatic Play Slot 2. Habanero Slot 3. PG SOFT Slot 4. Slot88 Slot 5. Spadegaming Slot 6. Microgaming Slot 7. PLAYTECH Slot!
Idol smoking site with blast game and great odds. The Sigari Bet site is one of the newly established sites that has been able to operate professionally in this short period of time. And it has attracted many users to this reliable site. The main reason for that is the explosive game. Below is the best odds on this Sigari Bet betting site. For this reason, the users of this site have reached millions of incomes. Sigari Bet has good features compared to rival sites, which makes this site special for users. Stay with us. If you want to enter the Bet smoking betting site without any trouble, you can enter the betting site from the green box above. Betting site: Sigaribet on the 16th of Adi Behesht, the name of this winner is Sigaribet, which has tried to diversify a new and standard experience of different betting for you. And the rewards are in front of you. Various questions about the sigaribet site. Sigaribat betting site games. In the two sports prediction sections with the largest number of betting sports and the casino section, which has 13 different games, including the explosion game; Monty, blackjack, poker, slot, backgammon, etc. Be sure to read: The explosion game + training and tricks of the explosion game are guaranteed to win.
Ensure Safety and Reliability with Verified Services from Muktu Mafia. In today’s digital landscape, ensuring the safety and reliability of online services is paramount. Whether you are betting on Sports Toto or looking for a secure online transaction, the risk of encountering scams is ever-present. This is where Muktu Mafia steps in, offering a trusted solution to keep you protected. Why Trust Muktu Mafia? Muktu Mafia is a renowned name in the world of scam verification, known for its rigorous review process and dedication to member safety. By implementing a thorough deposit system, we ensure that all transactions are secure, and members assets are safeguarded in case of emergencies. Comprehensive Verification Process: Our team conducts continuous monitoring and detailed inspections to verify the safety of each site we endorse. Only sites that pass our stringent criteria are recommended, providing you with a safe playground for all your betting needs. Member Protection: In the event of an emergency, our deposit system acts as a safety net, protecting your assets and giving you peace of mind. Our commitment to member safety has earned us the trust of thousands of users, making us the top choice for fraud verification. Expert Recommendations: Muktu Mafias experts leverage extensive data and years of experience to provide you with the best sites that offer secure and reliable services. We prioritize sites that excel in operational performance, user satisfaction, and security measures. Stay Informed with Muktu Mafia Beyond providing verified site recommendations, Muktu Mafia is dedicated to educating our members: Up-to-Date Information: Access the latest sports news, team statistics, and player conditions to make informed betting decisions. Educational Content: Learn effective betting strategies, risk management methods, and game analysis from our high-quality content. Continuous Support: Our team is always available to assist you with any queries or issues, ensuring you have the best support throughout your betting journey. Join Muktu Mafia Today Make the smart choice and join Muktu Mafia today to enjoy a secure, reliable, and enriching betting experience. Visit our website to explore our verified sites and start betting with confidence. Your safety and satisfaction are our top priorities, and we are committed to providing you with the best possible service.
Such sites are important because they provide a large dose of useful information. The most interesting text on this interesting topic can be found on the net. Beaver says I also have such interest, you can read my profile here. These things are very important, good think so - I think so too...
Mengikuti jejak para pemain berpengalaman, dengan odds tertinggi dan pilihan situs judi casino yang lengkap, Anda memiliki kesempatan emas untuk memilih strategi tepat guna meningkatkan peluang kemenangan Anda. Setiap situs menawarkan berbagai permainan menarik, mulai dari slot hingga permainan meja klasik, semuanya dirancang untuk memberikan pengalaman bermain yang maksimal. Memahami cara kerja setiap permainan dan memanfaatkan peluang terbaik bisa menjadi kunci kesuksesan Anda dalam dunia judi online.
Mulai petualangan slot Anda dengan Slot777 Login. Proses login yang cepat dan mudah memastikan Anda dapat segera bermain dan menikmati berbagai permainan slot seru dengan hadiah melimpah.
Nikmati sensasi bermain slot dengan BANDAR SLOT777 yang terkenal dengan Slot Gokil Rafigaming. Dengan peluang jackpot yang tinggi dan berbagai promosi serta bonus yang melimpah, situs ini menjadi pilihan utama bagi para pecinta slot.
I use only high-quality materials - you can see them at. Very interesting information, worth recommending. However, I recommend this. For many people this is important, so check out my profile:
For true fans of this thread, I will address it a free online. Can nicely write on similar topics! Welcome, here you'll find out how it should look. I invite you to the page where you can read interesting information on similar topics. I have a similar interest this is my page read everything carefully and let me know what you think.
I use only high-quality materials - you can see them at. Very interesting information, worth recommending. However, I recommend this. For many people this is important, so check out my profile:
I use only high-quality materials - you can see them at. Very interesting information, worth recommending. However, I recommend this. For many people this is important, so check out my profile:
Discover the Best Cannabis Products at Australian Organic Weed. Welcome to Australian Organic Weed, your premier destination for high-quality cannabis products. Our online shop offers a wide selection of top-notch marijuana strains, all available for discreet and secure purchase. Whether you are looking for Violator Kush, White Widow, or Super Silver Haze, we have something for everyone. Enjoy free shipping on orders over $100, ensuring you get the best value with every purchase. At Australian Organic Weed, customer satisfaction is our top priority. We provide 24/7 dedicated support to assist you at every step, ensuring a seamless shopping experience. If you are not completely satisfied with your purchase, our refund guarantee has you covered. Our products are carefully selected, tested, and packaged discreetly to ensure your privacy and safety. Join our community of happy customers who rave about our excellent service and premium products. Do not miss out on our exclusive offers and updates – subscribe to our newsletter today for the latest news and promotions. Shop with confidence and experience the best in cannabis products at Australian Organic Weed. Visit us online and discover why we are the trusted choice for cannabis enthusiasts in Adelaide and beyond. Shop now and elevate your experience!
Pilih Bandar Slot777 sebagai pilihan utama Anda untuk pembayaran cepat dan aman. Kami menyediakan sistem pembayaran yang handal dan efisien, memastikan setiap kemenangan Anda segera tersedia untuk dicairkan. Dukungan pelanggan yang ramah dan responsif menjadikan pengalaman bermain Anda lebih menyenangkan.
Csbola: Your Trusted Football Betting Partner for Euro 2024 Welcome to CSBOLA, the premier soccer betting site and proud partner of Euro 2024. At CSBOLA, we guarantee a secure and exceptional betting experience. With 24-hour service and a minimum deposit of IDR 25,000, you can enjoy hassle-free betting on your favorite teams. Our top-notch security ensures a safe environment for all players, while our attractive bonuses and promotions reward both new and loyal members. CSBOLA understands that players seek significant profits and an enjoyable gaming experience. Our site offers a variety of promotions and benefits, making it the go-to platform for soccer betting enthusiasts. Our expert team ensures a fair and transparent gaming environment, free from any cheating. We stand out as a trusted agent with unmatched services, including a 10% new member bonus, cashback options, easy deposits via electronic wallets, and a 3% referral bonus. With a diverse range of games, from online sportsbook betting to live casino experiences, CSBOLA caters to all your betting needs. Join CSBOLA today and experience the best in online soccer betting for Euro 2024!
Why Choose Byfavorites Cart? There are several key reasons Byfavorites carts stand out from the competition: High-Quality Ingredients: All Byfavorites vape products contain at least 95% hemp-derived Delta-8 THC oil and premium-grade terpenes. They use no cutting agents or other fillers that can degrade quality & potency. Third-Party Lab Tested: Every batch of every Byfavorites cart flavor and strain undergoes independent third-party testing at ISO-certified labs. This ensures safety, purity, potency, & compliance. Great Flavor Variety: Shoppers can choose from tantalizing flavors like Gelato, Zkittlez, Strawberry Cough, and Sour Diesel, along with effects-based options like Calm, Sleep, and Focus.
Listed here you'll learn it is important, they offer the link in a helpful webpage. Listed here you'll learn it is important, they offer the link in a helpful webpage. On this subject internet page, you'll see my best information, be sure to look over this level of detail. It's superior, however, check out the material at the street address. You should mainly be superior together with well-performing material, which means that see it:
Land Office: Your Trusted Partner in Safe Gaming. Welcome to Land Office, where trust and safety in online gaming are our top priorities. Our platform ensures that you can enjoy Toto and casino games without the fear of fraud. All our registered sites undergo rigorous verification, providing you with a secure and transparent gaming experience. As a new member, you receive a 100% guarantee against fraud when signing up using our exclusive membership code. We offer a seamless blend of Toto and casino games, alongside free money benefits and access to major site recommendations. Our events, like the ongoing Haechuk and KBO events, offer substantial prize money, enhancing your gaming excitement. At Land Office, we also provide free sports broadcasts, comprehensive fraud protection, and 24/7 customer support. Our commitment to safe and reliable services allows you to play confidently, knowing we have your back. Join our community today and experience a gaming environment built on trust and expertise. Enjoy the best of online gaming with Land Office!
TOTO PLAY AGENT: Your Ultimate Guide to Gacor Slots in 2024! Welcome to TOTO PLAY AGENT, your go-to platform for trusted and thrilling online slot experiences in 2024. With an official and trusted license, we offer a 20% deposit bonus to boost your chances of hitting big wins this year. Our site provides a secure and enjoyable environment for all players, ensuring a seamless gaming experience. Online slots have become a popular alternative to traditional gambling in Indonesia, especially during the COVID-19 era. The convenience of playing from your smartphone, combined with captivating graphics and easy gameplay, has made online slots a favorite pastime. At TOTO PLAY AGENT, we feature the top 10 online slots booming in Indonesia, including favorites like Gates of Olympus, Sweet Bonanza, and Wild West Gold. These games offer exciting features, high RTPs, and massive win potential. We also highlight trusted providers like Pragmatic Play, Playtech, and Microgaming, ensuring you have access to high-quality and fair games. Our platform is designed to give you the best gaming experience, complete with various bonuses and promotions. Join TOTO PLAY AGENT today, register, and take advantage of our 20% deposit bonus. let’s GACOR together and make 2024 your year of big wins!
Ligaklik: The Premier Online Soccer and Sports Agent in Asia. Welcome to Ligaklik, the number one online soccer and sports agent site in Asia. We pride ourselves on offering the most complete and exciting markets for soccer betting enthusiasts in Indonesia. As the leading parlay agent, Ligaklik ensures a safe, reliable, and enjoyable betting experience. At Ligaklik, we offer a minimum deposit and withdrawal of just 25 thousand, and you can place bets starting at only 10 thousand. Our platform allows you to choose from a variety of betting options, including parlay bets with a minimum of three teams and a maximum of ten teams. The more teams and higher the odds, the greater your potential winnings. We guarantee fast updates and quick withdrawals, with winnings deposited directly into your account within seconds. Our customer service team is available 24/7 to assist with any questions or issues. Deposits can be made through all major Indonesian banks, e-wallets like DANA, OVO, GOPAY, and LINK AJA, or even via mobile credits from XL and Telkomsel. Ligaklik offers various betting markets such as 1X2, HDP, Over/Under, and Odd/Even. With our exclusive bonuses, cashback offers, and referral programs, Ligaklik ensures you get the best value for your bets. Join Ligaklik today and experience the best in online soccer betting. Register now and enjoy secure, reliable, and exciting betting with Ligaklik!
Slot777 merupakan permainan Game Judi Slot Terlengkap dan Resmi di seluruh Indonesia.
Harvey777 merupakan agen game taruhan judi slot terlengkap dan terpopuler di seluruh Indonesia.
Rafigaming merupakan platform Game Slot Online Terbesar dan Terbaik di seluruh Indonesia.
Rafigaming Slot adalah platform Game Slot Online Terpercaya dan Teraman di seluruh Indonesia.
International Toto: Your Trusted Partner in Safe Betting. International Toto is a leading verification company dedicated to providing a secure and reliable betting environment. We specialize in ensuring that Toto and casino sites are thoroughly vetted, offering a fraud-free experience for users. Our robust verification system guarantees 100% compensation in the event of fraud, ensuring that your winnings are always protected. At International Toto, we prioritize user safety and satisfaction by offering a variety of betting options across major sports, including overseas soccer, baseball, and basketball. Our platform is designed to provide quick and accurate information, allowing users to enjoy real-time betting with confidence. In addition to sports betting, International Toto introduces transparent and trustworthy casino sites. We offer a wide range of games, including Baccarat, Blackjack, and slots, all backed by a commitment to fairness and security. Users can also benefit from various promotions, free money offers, and our casino payback program. By choosing International Toto, you’re not just betting—you’re betting with peace of mind. Join us today and experience the most reliable and secure platform for all your betting needs.
Land Office: Your Gateway to Secure Betting. The Land Office is committed to providing a secure and fraud-free betting environment for users. Our platform ensures that all Toto and casino sites undergo thorough verification, guaranteeing 100% compensation in case of any fraud. With our services, you can confidently enjoy a wide range of games, including Toto and casino games, while benefiting from free money offers. We take pride in recommending only the most reliable and safe playgrounds, where users can bet on popular sports events like KBO, MLB, NBA, and more. Our platform also offers free sports broadcasts and special events like the Haechuk and KBO events, where users can win substantial prizes. At the Land Office, we believe in transparency and user satisfaction. By signing up with our direct membership code, you gain access to our fraud protection program, ensuring a secure and enjoyable betting experience. Whether you are interested in sports betting or exploring various casino games, Land Office provides a trusted and verified platform where safety and reliability are guaranteed. Join us and enjoy a worry-free gaming experience today.
Casino & Slot Guarantee Company is a trusted certification authority in the online gaming community, dedicated to ensuring user safety and fraud prevention. we prioritize the safety and satisfaction of our users above all else. As a certified fraud verification company, we are dedicated to ensuring that our members can enjoy online casino and slot games without the fear of being scammed. Our commitment to transparency and fast resolution is unmatched, and we offer 100% compensation in the event of any fraudulent activity—provided that you sign up through a code from a major site verified by us. We only work with slot and casino sites that have passed rigorous fraud verification checks. By partnering with Casino & Slot, you are guaranteed a safe and reliable gaming experience on platforms that have proven their integrity. We understand that trust is crucial in the online gaming industry, which is why we go the extra mile to provide a secure environment for our users. At Casino & Slot, you can enjoy a wide variety of games, including popular slots and baccarat, with the peace of mind that comes from knowing you are playing on a verified platform. We also offer various bonuses and free money opportunities to enhance your gaming experience. Our dedication to user safety and our transparent, efficient service make Casino & Slot the ideal choice for those seeking a trustworthy online casino community.
Kazancın ve Eğlencenin Adresi: Betpark Dünyanın dört bir yanından bahis severlerin buluşma noktası olan Betpark, kazancın ve eğlencenin kapılarını ardına kadar açıyor. Spor bahislerinden canlı casino oyunlarına, pokerden sanal bahis seçeneklerine kadar uzanan geniş oyun yelpazesi ile Betpark, kullanıcılarına hem kazanç hem de sınırsız eğlence sunuyor. Spor Bahislerinde Yüksek Kazanç Fırsatları Spor tutkunuysanız, Betpark sizin için biçilmiş kaftan! Futboldan basketbola, tenisten motor sporlarına kadar çeşitli spor dallarında bahis yapma imkanı sunan Betpark, yüksek oranlarla kazanç elde etme fırsatı sunuyor. Maçları canlı takip ederken bahis yaparak heyecanın dozunu artırabilirsiniz. Canlı Casino Deneyimi Betpark’ın canlı casino bölümü, gerçek bir casinonun atmosferini evinize getiriyor. Gerçek krupiyeler eşliğinde blackjack, rulet, bakara gibi klasik oyunların keyfini çıkarabilirsiniz. Canlı yayınlanan oyunlarda şansınızı denerken aynı zamanda sosyal bir ortamda diğer oyuncularla da iletişim kurabilirsiniz. Güvenli ve Kullanıcı Dostu Platform Betpark, kullanıcılarına güvenli bir oyun ortamı sağlama konusunda titizlikle çalışıyor. Kişisel verilerinizin korunması ve ödemelerinizin güvenli bir şekilde gerçekleştirilmesi, Betpark’ın öncelikleri arasında yer alıyor. Ayrıca, kullanıcı dostu arayüzü sayesinde Betpark’ta oyun oynamak son derece kolay ve keyifli. Dikkat Edilmesi Gerekenler Bahis oyunları her ne kadar eğlenceli ve kazançlı olsa da, maddi kayıplara yol açabileceğini unutmamak gerekir. Bu yüzden, Betpark’ta yer alırken dikkatli olmalı, bütçenizi zorlamadan, keyif için küçük miktarlarla oynamalısınız. Sonuç Betpark, eğlence ve kazanç dolu bir dünyanın kapılarını açıyor. Siz de bu dünyaya adım atmak ve kazananlar kervanına katılmak istiyorsanız, Betpark’a hemen giriş yapın ve şansınızı deneyin!
Betgaranti ile Hayallerinizi Gerçekleştirin. Online bahis dünyasında fark yaratmak isteyenlerin tercihi Betgaranti, yüksek oranlar ve çevrimsiz bonuslar sunarak kullanıcılarına kazançlı bir deneyim vaat ediyor. Betgaranti nin sunduğu fırsatlar sayesinde, bahis severler hayallerini gerçeğe dönüştürebilirler. Site, spor bahislerinden canlı casino oyunlarına, pokerden sanal bahis seçeneklerine kadar geniş bir yelpazede hizmet sunuyor. Güvenilir ve Kolay Ödeme Yöntemleri Betgaranti, kullanıcılarına güvenli ve hızlı ödeme yöntemleri sunarak farkını ortaya koyuyor. Kredi kartı, banka havalesi, EFT, kripto para ve ön ödemeli kartlar gibi birçok seçenekle kullanıcılar, kazançlarını güvenli bir şekilde çekebilirler. Ayrıca, site üzerinden yapılan yatırımlarda ekstra freespin ve bonuslar kazanmak da mümkün. Betgaranti ile Canlı Bahis Heyecanı Betgaranti’nin canlı bahis bölümü, kullanıcılarına anında sonuç alabilecekleri bahis seçenekleri sunuyor. Gün boyunca farklı karşılaşmalar için açılan canlı bahisler, yüksek oranlarıyla dikkat çekiyor. Betgaranti, canlı bahislerde sunduğu oranlarla da rakiplerinden ayrılıyor ve kullanıcılarına kazançlı bir bahis deneyimi sunuyor. 7/24 Canlı Destek Betgaranti, kullanıcılarının karşılaşabileceği her türlü sorunu çözmek için 7/24 canlı destek hizmeti sunuyor. Deneyimli müşteri temsilcileri, site içi işlemler, bahisler ve bonuslar hakkında her türlü soruya anında yanıt vererek kullanıcı deneyimini en üst seviyeye çıkarıyor. Sonuç Betgaranti, yüksek oranlar, güvenilir ödeme yöntemleri ve kullanıcı dostu hizmetleri ile bahis dünyasında öne çıkıyor. Betgaranti’ye hemen üye olun, kazancın ve eğlencenin tadını çıkarın!
Welcome to Naga303: Your Ultimate Online Gambling Destination. Naga303 is the premier online gambling platform designed to offer you the most complete and thrilling gaming experience. As the most trusted and comprehensive online gambling site in Asia, we provide an extensive range of games, including lottery, slots, sportsbooks, and live casinos, catering to all types of players. At Naga303, we prioritize your safety and enjoyment. Our platform is built on advanced technology that guarantees 100% confidentiality and security for all your personal data. Whether you’re spinning the reels on popular slots like Gates of Olympus and Sweet Bonanza, placing your bets on your favorite sports teams, or participating in live casino games, you can do so with the confidence that your information is protected. We understand the importance of seamless transactions, which is why Naga303 offers the fastest deposit and withdrawal processes. With a minimum deposit of just Rp. 10,000 and withdrawals starting from Rp. 50,000, you can manage your funds with ease. Our 24/7 customer support is always ready to assist you, ensuring that your gaming experience is smooth and enjoyable. As a trusted site with millions of loyal members, Naga303 has earned a reputation for reliability and integrity. No matter how much you win, your withdrawals will be processed quickly and paid out in full. We are committed to providing you with the best odds and the most lucrative jackpots, giving you the chance to win big every time you play. Join Naga303 today and discover why we are the best choice for online gaming enthusiasts. Your journey to endless entertainment and incredible rewards starts here!
HUMASTOGEL adalah platform taruhan online terpercaya yang menyediakan berbagai jenis permainan seperti togel online, live casino, dan slot online. Dengan satu akun, pemain dapat menikmati akses mudah ke semua jenis taruhan ini, menjadikan HUMASTOGEL pilihan ideal bagi penggemar judi online. HUMASTOGEL telah mendapatkan lisensi resmi dari IDNPLAY dan PAGCOR, dua otoritas terkemuka dalam industri ini, yang memastikan keamanan dan kenyamanan dalam bermain. HUMASTOGEL juga dikenal sebagai situs togel online resmi terbesar dan terlengkap, dengan pasaran togel yang mengikuti hasil dari organisasi internasional seperti WLA (World Lottery Association) dan APLA (Asia Pacific Lottery Association). Pasaran populer seperti Togel Singapore, Togel Hongkong, dan Togel Sidney tersedia di platform ini, memberikan pengalaman bermain yang transparan dan adil. Selain togel, HUMASTOGEL juga menawarkan berbagai permainan slot online dari provider ternama seperti Pragmatic Play, Habanero, PG Soft, dan IDN Slot. Setiap provider menawarkan pengalaman bermain yang unik, dengan RTP (Return to Player) yang tinggi dan grafik yang menarik. Dengan layanan pelanggan 24/7 dan sistem keamanan yang terjamin, HUMASTOGEL memastikan pemain dapat menikmati permainan tanpa khawatir. HUMASTOGEL berkomitmen untuk memberikan pengalaman taruhan terbaik dan terpercaya, menjadikannya pilihan utama bagi para pemain di Indonesia.
Unik4D is a leading platform in the world of online lottery and 4D slots, officially launched in Thailand. Known for offering comprehensive information on popular lottery markets such as Singapore, Hong Kong, Macau, China, and Taiwan, Unik4D provides a secure and enjoyable experience for its players. The platform operates on advanced AI and cloud-based servers across Thailand, Vietnam, and Malaysia, ensuring smooth and reliable gameplay. Unik4D caters to both seasoned lottery enthusiasts and newcomers, offering a wide range of games with affordable betting options. With a starting bet of just 10 silver, players can explore various lotteries and online slots. The platform is backed by the most advanced security systems, offering a 97% safety rating in markets like Singapore and Hong Kong, making it a trusted choice for those seeking a fair and transparent gaming experience. In addition to its robust gaming options, Unik4D is dedicated to helping players maximize their success. It provides tips, tricks, and predictions to assist players in making informed decisions, ensuring a thrilling and rewarding experience. With 24/7 customer support, secure payment options through major banks and e-wallets, and a minimum deposit of just 5,000 rupiah, Unik4D offers a seamless and accessible entry into the world of online lottery and slot gaming.
Slotfafa88: The Ultimate Gacor Slot Site for Easy Wins. Slotfafa88 is a premier destination for online gambling enthusiasts seeking big wins and a thrilling slot experience. As a leader in the industry, Slotfafa88 offers a variety of slot games with innovative themes and displays that cater to both new and experienced players. The platform is designed to deliver high-quality entertainment while maximizing player satisfaction. At Slotfafa88, the mission is clear: provide generous jackpots and an easy-to-win environment for slot lovers. With jackpots ranging from tens of millions to hundreds of millions of rupiah, the site stands out as a top choice for those who want to enjoy the thrill of online gambling while also having the opportunity to earn substantial rewards. The platform’s advanced technology allows players to indulge in slot games from anywhere, at any time. Whether you are playing to unwind or aiming for big wins, Slotfafa88 ensures a seamless and enjoyable experience. If you ever encounter any issues, the 24/7 live chat support is available to resolve concerns and ensure a smooth gaming experience. Top Gacor Slot Games of 2024 for those eager to dive into the action, Slotfafa88 features a range of easy-to-win gacor slot games with impressive RTPs (Return to Player). Here are six recommended games that stand out this year: 1:> Slot88 (RTP 95.24%) 2:> Habanero (RTP 92.18%) 3:> Microgaming (RTP 91.55%) 4:> Pragmatic Play (RTP 90.78%) 5:> PG Slot (RTP 98.68%) 6:> Spadegaming (RTP 93.02%). Explore Slotfafa88 today and experience the excitement of playing the most gacor slots with easy winning strategies!
Empire88: Indonesia’s Leading Gacor Game Provider. Empire88 is a top-tier online game provider platform in Indonesia, offering a dynamic and rewarding gaming experience for enthusiasts of online gambling. Known for its extensive game selection and exceptional service, Empire88 has quickly established itself as a trusted name in the industry. At the core of Empire88’s appeal is its collection of gacor games with high Return to Player (RTP) rates, ensuring players have higher chances of winning. With games from leading developers such as Pragmatic Play, PG Soft, and Microgaming, players can enjoy a variety of immersive themes and innovative features. Empire88 makes transactions effortless with a range of payment options, including bank transfers, e-wallets like DANA, OVO, and GoPay, and QRIS payments. The platform ensures that all transactions are fast, secure, and encrypted for maximum safety. In addition to the excitement of gameplay, Empire88 provides generous bonuses and promotions, including a welcome bonus for new players and regular offers such as cashback, free spins, and lucrative tournaments. A dedicated 24/7 customer service team is available to assist with any questions or concerns. The platform is user-friendly, with a sleek and intuitive design that is optimized for mobile devices, allowing players to access their favorite games anytime, anywhere. With a loyalty program offering attractive rewards and a VIP program for frequent players, Empire88 is the ideal platform for those seeking thrilling gaming and substantial rewards. For a seamless and exciting online gaming experience, Empire88 is the perfect choice.
DrugHub Market: The New Frontier in Secure Online Transactions. DrugHub Market is the latest project from the creators of White House Market, renowned for its cutting-edge security and user-friendly design. DrugHub sets a new standard in online marketplaces by implementing robust features aimed at ensuring a safe and efficient experience for users and vendors alike. Enhanced Security Features At the heart of DrugHub is its unique access system designed to prevent phishing and DDoS attacks. Every user is assigned a dedicated .onion mirror, ensuring personalized and secure access to the platform. This mirror is verified against DrugHub’s PGP key, guaranteeing its authenticity. Vendors, in turn, are provided with permanent mirrors, streamlining their operations and interactions with buyers. User Registration and Authentication DrugHub incorporates a detailed registration process, which requires the use of PGP encryption. This not only safeguards user information but also adds an additional layer of security, preventing unauthorized access. Users authenticate themselves via PGP for each login, further reinforcing the market’s commitment to secure transactions. Efficient Product Search and Navigation Navigating DrugHub is effortless. The platform offers a simple and advanced search function, allowing users to find products based on a wide range of criteria. From sorting by price to filtering based on vendor ratings, the platform ensures that users can easily access the listings they are looking for. Streamlined Transactions with XMR DrugHub facilitates transactions using Monero (XMR), prioritizing anonymity. The platform’s checkout process is secure, requiring users to encrypt their order details with the seller’s PGP key, ensuring privacy and security throughout the purchase. DrugHub Market offers a well-rounded, secure, and user-friendly experience, continuing the legacy of its predecessor while improving upon it with new, cutting-edge features.
SLOT828 adalah situs slot online terkemuka di Indonesia yang menawarkan berbagai permainan slot gacor dengan tingkat kemenangan tinggi. Dengan pengalaman lebih dari 8 tahun, SLOT828 telah membangun reputasi sebagai platform terpercaya yang bekerja sama dengan agen resmi Slot88 untuk menyediakan game judi slot yang mudah dimenangkan. Sebagai salah satu situs terbesar, SLOT828 menawarkan beragam fitur unggulan, seperti bocoran RTP Slot Gacor dan komunitas pola slot, yang membantu meningkatkan peluang kemenangan para pemain. SLOT gacor hari ini menjadi semakin populer karena menawarkan tingkat kemenangan yang lebih tinggi dibandingkan permainan slot online lainnya. Di SLOT828, para pemain dapat menikmati game slot dengan RTP mencapai 98.47%, memberikan peluang kemenangan yang jauh lebih besar. Pengakuan dari lembaga pengamat judi online dunia, seperti PAGCOR, Commission Bet, dan Rebate Organization, memberikan lisensi resmi kepada SLOT828, memastikan keamanan dan keandalan situs ini. SLOT828 juga dikenal karena layanan pelanggan yang profesional dan responsif, siap melayani selama 24 jam untuk memastikan kenyamanan para pemain. Dalam hal transaksi, SLOT828 menawarkan berbagai metode yang memudahkan pemain, termasuk transfer bank, e-wallet, QRIS, dan deposit pulsa. Fleksibilitas ini membuat proses deposit dan penarikan dana menjadi lebih mudah dan cepat, sehingga pemain dapat fokus menikmati permainan. Jika Anda mencari situs slot gacor terpercaya yang menawarkan pengalaman bermain yang aman, nyaman, dan menguntungkan, SLOT828 adalah pilihan terbaik. Dengan berbagai keuntungan yang ditawarkan, SLOT828 memastikan setiap pemain memiliki peluang besar untuk meraih kemenangan besar setiap harinya. Bergabunglah sekarang dan nikmati sensasi bermain slot online di SLOT828.
SLOT828 adalah situs slot online terpercaya yang telah beroperasi selama 8 tahun dan menjadi salah satu pilihan utama para pemain judi online di Indonesia. Dengan pengalaman yang matang, SLOT828 telah menjalin kerja sama dengan agen resmi Slot88 untuk menyediakan permainan slot online yang mudah menang hari ini. Situs ini juga dilengkapi dengan fitur-fitur unggulan seperti bocoran RTP Slot Gacor serta komunitas pola slot, yang membantu para member meningkatkan peluang kemenangan mereka. Slot gacor di SLOT828 dikenal dengan tingkat kemenangan yang tinggi, mencapai RTP Slot88 hingga 98,47%. Hal ini menjadikan permainan di situs ini lebih menarik dan menguntungkan dibandingkan dengan situs judi slot lainnya. Berkat keunggulan tersebut, SLOT828 telah diakui oleh lembaga pengawas judi online dunia dan mengantongi lisensi resmi dari PAGCOR, Commission Bet, dan Rebate Organization, sehingga memberikan jaminan keamanan dan kredibilitas kepada para pemain. Selain menawarkan permainan yang gacor dan mudah menang, SLOT828 juga dikenal karena layanan Customer Service yang ramah dan siap membantu selama 24 jam. Situs ini menyediakan berbagai metode transaksi yang lengkap, termasuk transfer antar bank, E-wallet, QRIS, dan deposit melalui pulsa. Kemudahan transaksi ini memastikan pengalaman bermain yang lebih praktis dan efisien bagi setiap pemain. Dengan segala keunggulan yang ditawarkan, SLOT828 menjadi pilihan tepat bagi Anda yang mencari situs slot gacor terpercaya untuk bermain slot online dengan aman, nyaman, dan peluang menang yang tinggi. Jangan ragu untuk bergabung dan rasakan sendiri keseruan bermain di SLOT828!
Betgaranti, online bahis ve casino oyunları tutkunları için güvenilir ve keyifli bir platform sunmaktadır. Curaçao lisansı ile 2015 yılından bu yana faaliyet gösteren Betgaranti, spor bahislerinden slot oyunlarına kadar geniş bir oyun yelpazesi sunarak kullanıcılarına kazanç fırsatları sağlar. Yüksek oranlar, çevrimsiz bonuslar ve freespin fırsatları ile kazançlarınızı artırabilirsiniz. Platform, kullanıcılarına sunduğu promosyonlar ve bonuslarla fark yaratır. Hoş geldin bonusu, yatırım bonusları ve kayıp iade bonusları gibi avantajlarla oyun deneyiminizi zenginleştirirken, sunduğu mobil uyumlu arayüz sayesinde her yerden erişim imkanı sağlar. Güvenli ödeme yöntemleri ve hızlı para çekme işlemleri ile kullanıcı memnuniyetine büyük önem veren Betgaranti, 7/24 müşteri desteği ile de her zaman yanınızdadır. Eğer eğlence ve kazancın bir arada olduğu güvenilir bir platform arıyorsanız, Betgaranti doğru adres! Yüksek oranlar, çeşitli oyun seçenekleri ve cazip bonuslar ile Betgaranti’de kazanmaya hemen başlayın.
SBOBET888 is a globally recognized online gambling platform, known for its trustworthiness and security. As the parent site of the renowned Sbobet, SBOBET888 ensures a seamless and exciting betting experience. The platform stands out with its 24/7 customer service, offering players support anytime they need, making online gambling more accessible and safer. SBOBET888 has earned the trust of players in Thailand and abroad, thanks to its stable operations and reliable financial processes. Whether you are into sports betting, online casino games, or slots, SBOBET888 delivers a wide variety of gaming options to satisfy every player. The platform supports multiple devices, allowing you to enjoy the thrill of gambling on a computer, tablet, or smartphone without any hassle. Additionally, SBOBET888 provides enticing promotions, including a daily 5% refund on losses and VIP privileges for regular players. The platform also guarantees secure transactions, allowing users to deposit and withdraw funds quickly and easily, with a robust system that ensures peace of mind for all players. With a user-friendly interface and a commitment to customer satisfaction, SBOBET888 is the go-to choice for an unforgettable online gambling experience.
PRABUTOTO is a trusted and secure online gaming platform that provides an exciting and reliable environment for players. Known for its vast selection of games, PRABUTOTO offers something for everyone, whether you enjoy live games, slots, or lottery. The platform features top providers in the gaming industry such as Joker, Queenmaker, Pragmatic Play, PGSoft, and Habanero, ensuring that players have access to high-quality and entertaining games. PRABUTOTO is committed to providing a safe and secure gaming experience. The platform is fully licensed in Indonesia and adheres to strict legal guidelines to protect its users. All games are easy to play, offering the chance to win significant jackpots, which can reach hundreds of millions of rupiahs. With a wide variety of game choices and multiple banking options, including BCA, BNI, BRI, and Mandiri, PRABUTOTO ensures seamless transactions for deposits and withdrawals. Customer satisfaction is a top priority at PRABUTOTO. The platform offers 24/7 customer support, ensuring that players receive assistance whenever they need it. Whether you are a seasoned gamer or new to online gaming, PRABUTOTO’s user-friendly interface and professional service make it an ideal choice for anyone looking to enjoy the thrill of online gambling. Join today and experience the excitement of winning big with PRABUTOTO!
DekingLED is a leading manufacturer and supplier of LED strip lights, offering high-quality lighting solutions to clients worldwide. Established in 2014, DekingLED has built a strong reputation for delivering top-tier products, ranging from flexible SMD LED strips to LED downlights and aluminum profiles. The company serves a global market, including Europe, North America, Asia, and beyond, with a focus on innovation, quality, and customer satisfaction. With a state-of-the-art manufacturing facility in Shiyan Town, Guangdong Province, DekingLED has been certified by ISO 9001, CE, RoHS, and EMC, ensuring that all products meet international quality and safety standards. The company prides itself on its rigorous quality control process, with every product undergoing thorough testing before shipment. This commitment to quality has earned DekingLED a loyal customer base, particularly in regions where energy-efficient lighting is in high demand. DekingLED’s expertise extends to providing custom solutions for various applications, including interior decoration, commercial spaces, and architectural lighting. The company offers fast delivery and exceptional technical support to ensure clients receive the best service possible. Whether you are looking to buy bulk LED strip lights or need specific lighting solutions, DekingLED is your trusted partner for all your LED lighting needs.
MONTANA88: Situs Resmi Slot Online Terpercaya di Indonesia. MONTANA88 adalah salah satu platform judi online terkemuka di Indonesia yang menghadirkan beragam permainan slot, permainan kasino, poker, dan taruhan olahraga secara langsung. Sebagai situs resmi judi slot online, MONTANA88 telah menjadi pilihan utama bagi para pemain di Indonesia yang mencari pengalaman bermain yang aman, nyaman, dan menguntungkan. Dengan reputasi yang kuat dan layanan yang terpercaya, MONTANA88 terus menghadirkan inovasi dalam dunia permainan slot online. Platform ini menawarkan berbagai macam permainan slot yang berasal dari provider terkemuka seperti Pragmatic Play, Microgaming, PG Soft, Habanero, dan masih banyak lagi. MONTANA88 dikenal karena menyediakan permainan slot dengan RTP (Return to Player) yang tinggi, yang membuat peluang kemenangan lebih besar bagi para pemain. Selain itu, situs ini juga menawarkan berbagai macam bonus menarik yang dapat dinikmati oleh anggota baru maupun anggota lama, seperti bonus deposit, cashback, dan promo spesial lainnya. Tidak hanya terbatas pada permainan slot, MONTANA88 juga menawarkan berbagai jenis permainan lain seperti permainan kasino, poker, hingga taruhan olahraga langsung. Dengan layanan yang lengkap, para pemain dapat menikmati semua jenis permainan hanya dengan menggunakan satu akun. Ini memberikan kemudahan dan fleksibilitas bagi para pengguna untuk mengakses berbagai jenis taruhan kapan saja dan di mana saja. MONTANA88 juga sangat mengutamakan kepuasan pelanggan dengan menyediakan layanan dukungan 24 jam yang siap membantu kapan saja. Platform ini dilengkapi dengan teknologi keamanan yang canggih untuk memastikan privasi dan keamanan data pemain tetap terjaga. Semua transaksi, baik itu deposit maupun penarikan, diproses dengan cepat dan efisien, memastikan kenyamanan pemain dalam setiap langkahnya. Dengan berbagai keunggulan yang ditawarkan, MONTANA88 terus menjadi pilihan terbaik bagi para pecinta slot online dan judi di Indonesia. Platform ini bukan hanya memberikan pengalaman bermain yang seru, tetapi juga memberikan peluang nyata untuk meraih kemenangan besar dengan bonus dan permainan yang gacor setiap hari.
mpo777.fyi adalah sumber informasi terbaru untuk pemain slot online yang mencari pengalaman bermain yang aman, seru, dan menguntungkan. Situs ini menyediakan akses mudah dan cepat ke berbagai permainan slot online terpopuler dan terpercaya, menawarkan berbagai pilihan game menarik dan promo eksklusif yang dirancang untuk meningkatkan peluang kemenangan pemain. Sebagai tujuan utama bagi penggemar slot, mpo777.fyi menghadirkan panduan lengkap, tips bermain, serta berita terkini seputar dunia slot online. Di sini, pemain dapat menemukan informasi penting tentang strategi bermain, jackpot, dan berbagai promo yang tersedia, membantu mereka membuat keputusan terbaik saat bermain. Selain itu, mpo777.fyi juga memastikan bahwa semua informasi yang disajikan berasal dari platform yang terpercaya, sehingga keamanan dan kenyamanan pemain selalu terjaga. Tidak hanya memberikan ulasan tentang permainan slot, mpo777.fyi juga berfokus pada penyediaan akses eksklusif ke berbagai promosi yang menguntungkan, yang dirancang untuk memberikan keuntungan lebih besar bagi para pemain. Dengan antarmuka yang user-friendly dan informasi yang mudah diakses, mpo777.fyi menjadi situs yang tepat bagi siapa saja yang ingin memaksimalkan pengalaman bermain slot online. Kunjungi mpo777.fyi untuk menikmati berbagai permainan terbaik dan dapatkan informasi terbaru untuk meraih kemenangan besar.
SpeedTV is your ultimate destination for live sports streaming and up-to-date sports information. Offering comprehensive coverage of international sports events, including soccer, basketball, and more, SpeedTV provides free, real-time broadcasts to ensure fans never miss a moment of the action. Whether you are a casual viewer or a die-hard sports enthusiast, SpeedTV has something for everyone. In addition to live streaming, SpeedTV also features live scores, detailed sports analysis, and a vibrant community where users can discuss games, strategies, and share insights. The platform goes beyond just watching games; it helps fans stay connected and informed about their favorite teams and leagues. SpeedTV is also known for its commitment to user satisfaction and safety. Verified vendors are featured on the platform, providing users with secure and trustworthy services. Members can earn Speed Money, the platform’s reward points, by actively engaging through comments, posts, and participating in daily logins. These points can be redeemed for various benefits within SpeedTV’s partner network, adding extra value to the user experience. Join SpeedTV today to enjoy hassle-free sports streaming and become part of a dynamic community of sports lovers!
Welcome to Dark Matter Market Wiki: A Secure Marketplace for Privacy-Conscious Users Since its launch in July 2022, Dark Matter Market has quickly become a trusted name in the darknet community, thanks to its user-friendly design and enhanced privacy features. Offering a wide range of products, from digital goods to cannabis and psychedelics, the platform is designed for users who prioritize anonymity and security. By exclusively supporting Monero (XMR) for transactions—a cryptocurrency renowned for its untraceable qualities—Dark Matter Market ensures a private, secure transaction environment. To further protect users, Dark Matter Market implements advanced security measures like walletless payments and multi-signature (multisig) transactions, reducing the risk of fund loss. Two-factor authentication (2FA) adds an extra layer of security, ensuring account protection even in case of password breaches. For secure messaging, Pretty Good Privacy (PGP) encryption is encouraged for vendor-buyer communication. Dark Matter Market’s intuitive interface makes it easy to navigate, with clearly defined categories and a powerful search function. Filters allow users to sort listings by factors like origin, destination, and vendor reputation. Additionally, the platform's Academy section provides tutorials on using PGP and multisig, empowering users to manage their security. Overall, Dark Matter Market is an innovative marketplace prioritizing user safety and privacy. With a focus on Monero transactions and strict vendor guidelines, it offers a dependable choice for those seeking a secure darknet experience.
Welcome to LoX Salon in the heart of Downtown Winchester, Virginia! At LoX Salon, we’re more than just a hair salon; we’re a community of licensed, passionate hair artists dedicated to customer service, education, and exceptional results. Our location at 209 E Boscawen Street is just a short walk from the historic district, making it convenient for our clients to experience the vibrant local area. Our team, TeamLoXVA, specializes in an array of services, including precision haircuts, advanced coloring, perms, and multicultural hair care. Whether you’re looking for a simple trim or an intricate color transformation, our hair artists bring expertise and creativity to every service. We’re also proud to be an environmentally friendly salon, using only safe, ammonia-free, and formaldehyde-free products to ensure a safe experience for both our staff and clients. In addition to hair services, we offer nail and facial waxing treatments. Planning a special event? We provide both in-salon and on-location styling for weddings, proms, and other memorable occasions. Our salon is fully accessible, with parking available for your convenience. While appointments are recommended, we welcome walk-ins whenever possible. Visit LoX Salon and let our talented team transform your hair, making every visit a unique experience. For us, life’s too short for boring hair – we invite you to join us in embracing a vibrant and confident look!
GACOR88: Situs Slot Online Terpercaya dengan RTP Slot Gacor Tertinggi di Indonesia. GACOR88 adalah platform slot online terpercaya di Indonesia yang menawarkan pengalaman bermain menguntungkan dengan koleksi permainan Slot88 terlengkap dan RTP (Return to Player) tinggi. Didedikasikan untuk memberikan peluang kemenangan besar bagi para pemain, GACOR88 didukung oleh teknologi keamanan terkini dan bekerja sama dengan provider ternama seperti Pragmatic Play, Habanero, dan PG Soft. Setiap permainan di GACOR88 dilengkapi fitur menarik, termasuk free spins, jackpot progresif, dan berbagai bonus lain untuk memaksimalkan peluang kemenangan. Sebagai situs resmi Slot88, GACOR88 menekankan pada keamanan dan transparansi dalam setiap transaksi dan permainan. Dengan sistem enkripsi canggih, data dan privasi pemain terlindungi secara maksimal, memberikan rasa aman selama bermain. Lisensi resmi yang dimiliki juga memastikan bahwa operasional situs ini sah dan dapat diandalkan oleh para pemain di Indonesia, sehingga mereka bisa menikmati permainan dengan tenang. Untuk kenyamanan transaksi, GACOR88 mendukung berbagai metode pembayaran lokal, seperti transfer bank dan e-wallet populer seperti OVO, DANA, dan GoPay. Proses deposit dan penarikan dana cepat dan aman, sehingga pemain dapat menikmati permainan tanpa hambatan. Selain itu, GACOR88 menyediakan layanan dukungan pelanggan 24/7 yang dapat diakses melalui live chat, WhatsApp, atau email. Tim dukungan yang profesional dan responsif siap membantu menjawab setiap pertanyaan atau menangani masalah dengan cepat. Dengan fokus pada keamanan, kenyamanan, dan RTP tinggi, GACOR88 menawarkan pengalaman bermain slot yang menyenangkan dan menguntungkan bagi para pemain di Indonesia.
DEWACUKONG88: Situs Slot Gacor Terpercaya dengan Peluang Maxwin Terbaik. DEWACUKONG88 adalah platform slot online terpercaya yang menghadirkan pengalaman bermain terbaik dengan koleksi game slot dari berbagai provider ternama seperti Slot88, PG Soft, dan Pragmatic Play. Situs ini dirancang khusus bagi para penggemar slot gacor yang mencari kemenangan besar dengan peluang maxwin. Dengan ribuan game slot berkualitas dari provider terbaik di dunia, DEWACUKONG88 menjadi destinasi utama bagi pemain yang ingin merasakan sensasi jackpot dengan peluang menang tinggi. Sebagai situs slot gacor terpercaya, DEWACUKONG88 memastikan keamanan dan kenyamanan pemain melalui sistem keamanan canggih yang melindungi data dan transaksi pengguna. Setiap permainan yang tersedia di DEWACUKONG88 telah melalui proses pemeriksaan ketat, bekerja sama dengan PACGOR untuk menjamin permainan yang adil dan transparan. Hal ini membuat pemain dapat menikmati permainan tanpa rasa khawatir, dengan proses withdraw cepat dan aman untuk setiap kemenangan. Selain keamanan, DEWACUKONG88 juga unggul dalam pelayanan. Tim dukungan pelanggan yang profesional siap melayani pemain 24/7, menangani setiap keluhan, dan memberikan informasi terbaru tentang bonus, promo, dan RTP slot terbaik. Fitur ini menjadikan DEWACUKONG88 sebagai situs yang tak hanya mengutamakan kemenangan, tetapi juga kepuasan pemain dalam setiap sesi bermain. Bagi pemain yang ingin mencoba peruntungan dan meraih kemenangan besar, DEWACUKONG88 adalah pilihan tepat. Dengan reputasi terpercaya, keamanan maksimal, dan berbagai opsi slot gacor yang mudah jackpot, DEWACUKONG88 siap membantu Anda meraih kesuksesan dalam bermain slot dan menikmati keseruan menjadi sultan slot.
Onca Play is a trusted platform providing users with a reliable guide to safe online gaming experiences, especially in baccarat. Featuring a thoroughly vetted list of the top 10 recommended baccarat sites, Onca Play ensures that users find platforms free from scams, frauds, and other risks. Each site in their curated list undergoes rigorous checks for safety, which include reviews of their operational history, licensing credentials, and user feedback to guarantee secure gameplay. Highlighted options on Onca Play include major platforms like Up Casino, Ten Casino, Bolt Casino, and Sonic Casino. These sites not only offer diverse baccarat game options, including live dealer experiences, but also provide exclusive bonuses like welcome credits and deposit match events to enrich the gaming experience. Players benefit from high-definition streaming, various betting options, and rapid transaction processing, making it convenient to enjoy high-quality baccarat from home. Onca Play goes a step further by educating users on key considerations when selecting baccarat sites, including SSL security, international licensing, and strong privacy practices. The platform also highlights reputable payment methods and provides ongoing security updates to ensure that players’ data remains protected. Through user reviews and community insights, Onca Play enables informed choices for safe and enjoyable online gaming experiences. Whether you are a beginner or a seasoned player, Onca Play offers a comprehensive guide to navigating online baccarat safely.
Onca Play – The Ultimate Guide to Safe and Verified Online Casino Sites in 2024. Onca Play stands out as a trusted community platform dedicated to helping users find secure, verified online casinos. Featuring a carefully curated list of top 10 casino sites, Onca Play uses strict criteria to recommend only the most reliable platforms. Each site undergoes a thorough verification process, ensuring they hold genuine licenses, offer SSL encryption, and adhere to fair gaming standards. Users can confidently explore Onca Play’s recommendations, knowing they’ve been vetted for security, fast transactions, and data protection. Onca Play’s recommended casinos offer an array of popular games, including high-quality live baccarat, interactive slots, and poker, giving players diverse entertainment options. Additionally, these platforms frequently feature bonus incentives, such as deposit match bonuses and no-risk credit (꽁머니), allowing players to maximize their experience. The high-definition streaming of live dealer games brings an authentic casino experience directly to users’ screens. Through user reviews and community insights, Onca Play provides detailed feedback on each casino, highlighting advantages like swift deposits, robust customer support, and mobile-friendly interfaces. By following Onca Play’s safety guidelines and benefiting from its promotions, players can enjoy a secure, dynamic online gaming experience. For anyone seeking a verified, safe casino site in 2024, Onca Play offers an invaluable resource.
OncaPlay is a trusted casino community platform that provides a list of the top 10 verified slot sites. As an essential resource for users seeking a safe online gaming experience, OncaPlay conducts thorough evaluations of each recommended site. The platform emphasizes selecting sites that have passed strict "eat-and-run" (scam) verification to ensure player protection. Among the recommended sites, users will find options offering a range of game types, including free slot trials, Pragmatic slots, and video slots. OncaPlay’s evaluation process includes analyzing each site’s security features, SSL encryption, and user reviews, selecting only sites with strong reputations for transparency, reliable payout processes, and high-quality customer service. Through detailed guides, OncaPlay also educates users on using bonus opportunities and secure deposit and withdrawal methods. By following these guides, players can maximize their gaming experience and minimize risks. For both beginners and seasoned players, OncaPlay provides the assurance needed to enjoy online slot games in a safe and supportive environment, empowering users to make well-informed choices on verified platforms.
تبادل جيست بوست
نحن نمتلك 17 رابط دوفلو ذات جودة عالية
من يرغب تبادل الروابط الخلفية النصية
يرجي التواصل معنا عبر
00491634511222 الواتس اب
https://www.rauhane.net/
https://www.elso9.com
https://www.s3udy.org
https://www.eljnoub.com
https://www.q8yat.org
https://hurenberlin.com
https://wikimedia.cc
http://www.alfalaki.net
https://www.jeouzal.org/
https://www.jaouzal.org/
https://www.sheikhrohani.de
https://www.myemairat.de
https://www.saudieonline.de
https://www.nejetaa.de
https://www.iesummit.de
https://www.jalbalhabeb.de
https://www.alukah.de
https://www.mqaall.de
https://www.elbalad.de
https://www.muhtwa.de
https://www.mawdoo3.de
https://casinoberlin.eu
https://jalbalhabeb.org/
https://telegra.ph/kiki-09-20-3
https://telegra.ph/BERLINintim-09-20
https://rauhane11.hashnode.dev/dor-shykh-rohany-fy-almgtmaa
https://rauhane112.blogspot.com/2024/09/blog-post_23.html
#زواج_السعودية #شيخ_روحاني_مغربي #شيخ_روحاني_معالج #شيخ_مغربي #شيخ_روحاني #السعودية_جدة #طلاسم #السعودية_الرياض #خواتم_روحانيه #ال_سعود #الرياض #الدمام #جدة #القطيف #القصيم #زواج_العوانس #رد_المطلقة #فتح_نصيب_العانس #تبوك #ينبع #سعودي #سعوديات #بنات_جده #بنات_الرياض #جلب_الحبيب #فك_السحر #زواج_العانس #جلب_الحبيب_للزواج
#جلب_الحبيب #جلب_الحبيب_للزواج #فك_السحر
#رد_المطلقة #سوق_واقف #الامارات #الكويت #البحرين #سلطنة_عمان #الرياض #عجمان #الفجيره #جدة #الطائف #كتارا_قطر
#شيخ_روحاني_مغربي #شيخ_روحاني #شيخ_روحاني_مضمون
#جلب_الحبيب
#شيخ_روحاني
#جلبالحبيب
#اغاني_خليجيه
#شيخة_روحانية
#شيخ_مغربي
#شيخة_مغربية
#الكويت
#الامارا
ت
#قطر
#محبة
#محبة_وتهييج
#سلب_ارادة
#جلب_الزوج
#رد_المطلقة
#رد_الزوج
#رد_الزوجة
#مكياج
#تجميل
#الرياض
#جدة
#الخبر
Buy fake ID cards online to enjoy the beauty of life at its best If you have lost your ID, it is unbearable to wait until state-run institutions issue a new one for you. Sometimes it feels like this moment will never come. Think of those embarrassing situations when you are booted out of a nightclub simply because your card is not ready, and you look too young. Well, gone are the days when you had to break your piggy bank to purchase some blurry piece of laminated paper that you could reproduce on a printer on your own. Take it easy as you can get a fake scannable ID of unmatched quality and at a rock-bottom price with octagonfakies! It is just a matter of days and a little bit of patience. Today’s market of counterfeit documents is on an unprecedented rise. That makes sense as the latest technologies enable you to end up having an identity document that is on a par with a real one. Octagonfakies is your most reputable supplier of fake ID cards and driver’s licences that are genuine-like. Don’t fall for some sketchy guys and stop paying a fortune for forged papers!. We do many kinds of ID products well, such as DL, SSN, Vaccine card, and the products cover all states in the United States. The best thing is that our ID versions are always updated by the newest real ones, if you need older versions, please contact us before placing an order. The production process is operated under strict QC standards to ensure that every card from here is 100% quality and speed as promised to our customers. Hopefully, more hits are coming: British IDs, Australian IDs, New Zealand IDs, Canadian IDs, , Graduation Certificate, Vaccine Card, Green Card, Passport, etc.
Welcome to a trusted guide for choosing verified and safe Toto sites, where security and enjoyable betting experiences come together. Our platform introduces top-rated major sites and sports Toto options, ensuring you can place bets confidently without the risk of fraud. Featuring expertly curated recommendations from verification specialists like MuktuFriends, we provide an exclusive list of trusted playgrounds with robust security measures and transparent operations. Here, you’ll discover key insights into the differences between sports Toto and major sites, as well as the essential criteria for building a secure betting environment. Verified by MuktuFriends, sites like Rulabet, DoubleUbet, and OneTopBet offer a wealth of options, from diverse sports betting to competitive casino games, catering to all preferences. Equipped with detailed reviews, user testimonials, and advanced fraud prevention measures, we help you navigate the betting world with confidence. Whether you’re drawn to the regulated sports Toto system or privately operated major sites verified for safety, our platform ensures a seamless experience. Dive into our comprehensive resources, which highlight the importance of thorough Muktu verification, strong financial systems, and user-friendly interfaces, to make informed choices for secure, exciting betting adventures. With us, the best Toto experience awaits you.
Ensuring Online Safety with Trusted Scam Verification. In today’s digital landscape, online safety has become a growing concern. With countless websites emerging daily, the risk of encountering fraudulent platforms is higher than ever. That’s where we come in—your trusted partner in scam verification and prevention. Our mission is to protect you from online scams by thoroughly analyzing and reviewing websites for their credibility. We specialize in identifying suspicious and risky platforms, ensuring you have the tools to navigate the internet safely. Through our advanced verification process, we evaluate websites against stringent safety standards, flagging fraudulent activities and recommending reliable alternatives. Transparency and accuracy are at the heart of our services. Our platform provides detailed insights and reports, empowering you to make informed decisions about where to engage online. Whether you’re shopping, browsing, or conducting business, we help safeguard your activities, offering peace of mind in a complex digital world. In addition to site evaluations, we are committed to educating users about the signs of scams and the best practices for staying secure. By raising awareness, we aim to create a safer online environment for everyone. Say goodbye to online fraud and hello to trustworthy digital experiences. With our expertise, you can explore the web confidently, knowing your safety is our top priority. Trust us to guide you to secure platforms and protect you from scams every step of the way.
Ensuring Online Safety with Trusted Scam Verification. In today’s digital landscape, online safety has become a growing concern. With countless websites emerging daily, the risk of encountering fraudulent platforms is higher than ever. That’s where we come in—your trusted partner in scam verification and prevention. Our mission is to protect you from online scams by thoroughly analyzing and reviewing websites for their credibility. We specialize in identifying suspicious and risky platforms, ensuring you have the tools to navigate the internet safely. Through our advanced verification process, we evaluate websites against stringent safety standards, flagging fraudulent activities and recommending reliable alternatives. Transparency and accuracy are at the heart of our services. Our platform provides detailed insights and reports, empowering you to make informed decisions about where to engage online. Whether you’re shopping, browsing, or conducting business, we help safeguard your activities, offering peace of mind in a complex digital world. In addition to site evaluations, we are committed to educating users about the signs of scams and the best practices for staying secure. By raising awareness, we aim to create a safer online environment for everyone. Say goodbye to online fraud and hello to trustworthy digital experiences. With our expertise, you can explore the web confidently, knowing your safety is our top priority. Trust us to guide you to secure platforms and protect you from scams every step of the way.
GACOR88BET: Platform Slot Online Gacor dengan Peluang JP Maxwin. GACOR88BET adalah pilihan utama bagi Anda yang mencari situs slot online terpercaya dengan tingkat kemenangan tinggi. Sebagai platform unggulan, kami menyediakan berbagai permainan slot gacor yang mudah dimainkan dan dirancang untuk memberikan peluang jackpot (JP) besar setiap hari. Proses pendaftaran di GACOR88BET sangat mudah, memungkinkan pemain baru untuk segera bergabung dan menikmati pengalaman bermain yang memuaskan. Kami bekerja sama dengan berbagai provider terkemuka seperti Pragmatic Play, Slot88, PG Slot, Spadegaming, Microgaming, dan Joker123. Semua provider ini terkenal dengan permainan berkualitas tinggi yang sering menghadirkan kemenangan besar bagi pemainnya. Untuk mendukung pengalaman bermain yang transparan, kami menyediakan RTP Live yang diperbarui secara real-time, sehingga pemain dapat memantau peluang kemenangan mereka dengan mudah. Dengan lisensi resmi dari PAGCOR, GACOR88BET menjamin keamanan, keadilan, dan integritas dalam setiap permainan. Selain itu, kami menawarkan berbagai event dan promosi menarik, termasuk hadiah progresif hingga 188 juta rupiah, yang dirancang untuk meningkatkan peluang kemenangan Anda. Di GACOR88BET, kepuasan dan kepercayaan pemain menjadi prioritas kami. Tim layanan pelanggan profesional kami siap membantu Anda 24/7, memastikan setiap kendala diselesaikan dengan cepat. Kami juga menyediakan fitur akun demo, memungkinkan pemain baru untuk mempelajari pola permainan sebelum bermain dengan uang sungguhan. Kami mengingatkan pemain untuk bermain secara bijak dan menjadikan permainan slot sebagai hiburan semata. Dengan peluang menang yang tinggi, dukungan teknologi canggih, dan berbagai bonus menarik, GACOR88BET adalah tempat terbaik bagi Anda untuk meraih kemenangan besar setiap hari. Bergabunglah sekarang dan nikmati pengalaman bermain slot online yang menguntungkan bersama GACOR88BET!
RumahBola: Agen Judi Bola Mix Parlay dan Situs Slot Online Terpercaya di Indonesia. RumahBola adalah agen judi bola mix parlay dan situs slot online terpercaya yang menyediakan pasaran bola terlengkap di Indonesia. Dengan pengalaman panjang di dunia taruhan online, RumahBola menawarkan platform yang aman, nyaman, dan berkualitas tinggi untuk semua pemain, baik pemula maupun profesional. Sebagai situs taruhan bola resmi, RumahBola menyediakan berbagai jenis taruhan, termasuk taruhan mix parlay, yang populer di kalangan pecinta olahraga. Selain itu, RumahBola juga menjadi tempat terbaik untuk bermain game slot online, poker, live casino, sabung ayam, dan berbagai permainan lainnya. Setiap permainan dilengkapi dengan grafik berkualitas tinggi, pengalaman bermain yang interaktif, dan tingkat kemenangan yang menguntungkan. RumahBola memiliki keunggulan dalam memberikan fasilitas lengkap bagi pemain, seperti minimal deposit yang terjangkau hanya Rp 25.000,-, metode pembayaran fleksibel melalui bank lokal dan e-wallet (Dana, OVO, Gopay, LinkAja), serta bonus menarik seperti cashback mingguan dan promo new member. Selain itu, pemain dapat menikmati layanan live streaming gratis untuk berbagai pertandingan sepak bola, termasuk liga top dunia dan Liga 1 Indonesia. Keamanan dan kenyamanan adalah prioritas utama di RumahBola. Dengan sistem teknologi canggih dan layanan pelanggan 24 jam, RumahBola siap membantu semua kebutuhan pemain kapan saja. RumahBola juga memastikan semua kemenangan dibayarkan langsung ke rekening pemain tanpa kendala, menjadikannya salah satu agen judi bola terpercaya di Indonesia. Bagi Anda yang ingin menikmati taruhan bola dan slot online dengan kualitas terbaik, RumahBola adalah pilihan tepat. Nikmati pengalaman bermain yang aman, nyaman, dan penuh keuntungan hanya di RumahBola! Bergabunglah sekarang dan buktikan sendiri kualitas layanan terbaik kami.
Topanwin: Situs Slot Gacor Online Terpercaya di Indonesia. Topanwin adalah platform judi online terpercaya yang menghadirkan berbagai permainan slot gacor terbaru dengan layanan 24 jam nonstop. Sebagai situs slot online terpercaya, Topanwin menyediakan pengalaman bermain yang seru, aman, dan menguntungkan bagi para penggemar slot di seluruh Indonesia. Dengan berbagai jenis permainan slot yang tersedia, pemain memiliki peluang besar untuk meraih jackpot besar dan maxwin hanya dengan modal kecil. Permainan slot online semakin populer berkat sensasi dan keseruannya. Di Topanwin, Anda dapat menikmati berbagai jenis slot gacor yang dirancang untuk memberikan pengalaman terbaik. Selain itu, setiap pemain memiliki kesempatan besar untuk mendapatkan hadiah fantastis yang dapat mengubah hidup mereka. Dengan strategi yang tepat dan sedikit keberuntungan, siapa pun bisa menjadi pemenang di Topanwin. Keamanan pemain menjadi prioritas utama Topanwin. Dengan sistem enkripsi canggih, semua data dan transaksi dijamin aman sehingga pemain dapat bermain dengan nyaman tanpa khawatir. Selain itu, kami menyediakan berbagai metode pembayaran, termasuk e-wallet seperti Dana, OVO, dan Gopay, yang memudahkan pemain untuk melakukan deposit dan penarikan dengan cepat. Topanwin juga menawarkan berbagai promo menarik, termasuk bonus cashback, bonus tanpa turnover (TO), dan program referral. Dengan mengajak teman untuk bergabung, Anda bisa mendapatkan penghasilan tambahan melalui bonus referral. Kami juga memastikan bahwa setiap kemenangan akan dibayarkan penuh tanpa hambatan, menjadikan Topanwin sebagai pilihan terbaik bagi para pemain. Bergabunglah sekarang di Topanwin, situs slot online gacor terpercaya yang memberikan layanan terbaik di Indonesia. Nikmati keseruan bermain, raih kemenangan besar, dan jadilah bagian dari komunitas Topanwin untuk pengalaman judi online yang tak terlupakan!
Telegram: Revolutionizing Communication Across Platforms. Telegram is a globally renowned messaging platform that seamlessly blends speed, security, and versatility to offer users a superior communication experience. Designed with cutting-edge technology, Telegram enables individuals and businesses to connect across various devices, including PCs, mobile phones, and web browsers. Whether you are downloading the latest version of Telegram for Windows, macOS, iOS, Android, or Linux, the platform ensures a consistent and smooth experience. One of Telegram’s key highlights is its commitment to privacy and security. By leveraging advanced encryption protocols, Telegram guarantees that your messages, voice calls, and shared media remain confidential. The platform also allows users to activate "Secret Chats," offering end-to-end encryption for added protection. This robust approach has earned Telegram a reputation as one of the most secure messaging apps available. Beyond its security features, Telegram’s functionality is designed to cater to a wide range of user needs. From sending text messages and multimedia files to engaging in large group chats with up to 200,000 members, the app supports both personal and professional communication. Telegram also introduces unique features such as customizable stickers, bots for automation, and channels for broadcasting information to unlimited audiences. For users exploring the platform for the first time, Telegram provides comprehensive tutorials, FAQs, and guides on its official website. The resources cover everything from installation and file management to advanced customization and troubleshooting. Whether you are looking to download Telegram’s Chinese version, web version, or mobile application, the process is quick and user-friendly. Join the millions of users worldwide who rely on Telegram for its unmatched combination of speed, reliability, and security. Download the app today from the official Telegram website and experience a new standard in modern communication.
HOKIZEUS88: Situs Slot Gacor Zeus & Link RTP Slot Online Terpercaya. HOKIZEUS88 adalah salah satu platform judi slot online terpercaya yang dikenal luas sebagai situs slot gacor terbaik di Indonesia. Dengan reputasi yang terus terjaga, HOKIZEUS88 menawarkan pengalaman bermain slot online yang seru, aman, dan memberikan peluang besar untuk memenangkan jackpot maxwin. Situs ini dilengkapi dengan berbagai fitur canggih yang memudahkan pemain menikmati permainan slot online kapan saja dan di mana saja. HOKIZEUS88 menyediakan metode transaksi yang modern, seperti Qris (QR Code), transfer bank, e-wallet, dan deposit pulsa. Semua transaksi diproses dengan cepat dan aman, sehingga pemain tidak perlu khawatir mengenai dana mereka. Keunggulan utama HOKIZEUS88 adalah kerja sama dengan provider slot online terkemuka, seperti Pragmatic Play, Slot88, dan PG Soft. Provider ini dikenal dengan permainan berkualitas tinggi yang sering memberikan kemenangan besar dan pengalaman bermain yang memuaskan. Selain itu, situs ini juga menawarkan bocoran live RTP slot yang akurat, membantu pemain memilih permainan dengan peluang kemenangan terbaik setiap harinya. HOKIZEUS88 tidak hanya menghadirkan permainan slot berkualitas, tetapi juga memberikan pelayanan pelanggan 24/7 yang responsif dan profesional. Dengan dukungan penuh dari tim customer service, pemain dapat merasa nyaman dan fokus menikmati permainan. Bagi Anda yang mencari sensasi bermain slot online dengan peluang besar dan akses mudah, HOKIZEUS88 adalah pilihan tepat. Nikmati berbagai permainan slot gacor Zeus dengan peluang kemenangan tinggi dan jadilah bagian dari komunitas pemain yang sukses. Bergabunglah sekarang dan rasakan pengalaman bermain slot online terbaik bersama HOKIZEUS88.
Leave a Comment